
 Copy to clipboard
Copy to clipboard 
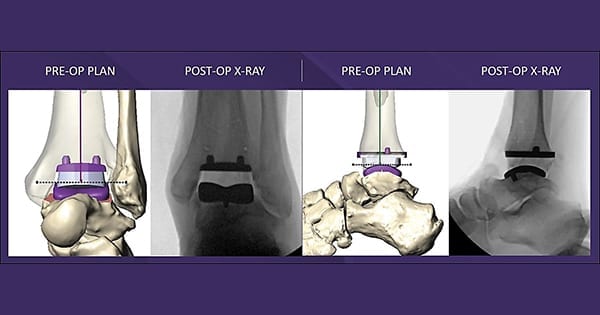
Paragon 28 received FDA 510(k) clearance to market the MAVEN™ PSI (Patient-Specific Instrumentation) system, a platform technology designed to expedite accurate positioning of the APEX 3D™ Total Ankle. The first case using the system has been completed.
MAVEN PSI includes features to promote alignment accuracy, stability and optimal implant placement. It is compatible with weight-bearing and traditional CT scanning technologies, and employs one continuous scan from the knee through the base of the foot to capture biomechanically accurate data.
MAVEN PSI includes three anatomically-contoured alignment guides, bone models and a customized pre-operative plan. The System features the first Tibial AP Positioning Spacer on the market, developed to simplify the sizing evaluation process of the tibial component and establish optimal tibial bone coverage. MAVEN PSI also allows for refined intra-operative positioning and micro adjustments, if necessary.
Paragon 28 received FDA 510(k) clearance to market the MAVEN™ PSI (Patient-Specific Instrumentation) system, a platform technology designed to expedite accurate positioning of the APEX 3D™ Total Ankle. The first case using the system has been completed.
MAVEN PSI includes features to promote alignment accuracy, stability and optimal...
Paragon 28 received FDA 510(k) clearance to market the MAVEN™ PSI (Patient-Specific Instrumentation) system, a platform technology designed to expedite accurate positioning of the APEX 3D™ Total Ankle. The first case using the system has been completed.
MAVEN PSI includes features to promote alignment accuracy, stability and optimal implant placement. It is compatible with weight-bearing and traditional CT scanning technologies, and employs one continuous scan from the knee through the base of the foot to capture biomechanically accurate data.
MAVEN PSI includes three anatomically-contoured alignment guides, bone models and a customized pre-operative plan. The System features the first Tibial AP Positioning Spacer on the market, developed to simplify the sizing evaluation process of the tibial component and establish optimal tibial bone coverage. MAVEN PSI also allows for refined intra-operative positioning and micro adjustments, if necessary.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







