Copy to clipboard
Copy to clipboard 
PainTEQ closed $35 million in non-dilutive growth capital backed by its intellectual property portfolio from a lending vehicle managed by MVolution Partners.
Net proceeds from this IP-backed debt financing will be used to expand the commercialization of LinQ, PainTEQ’s implantable sacroiliac (SI) joint stabilization system, as well as support research and development efforts aimed at advancing care for patients with SI joint pain and dysfunction.
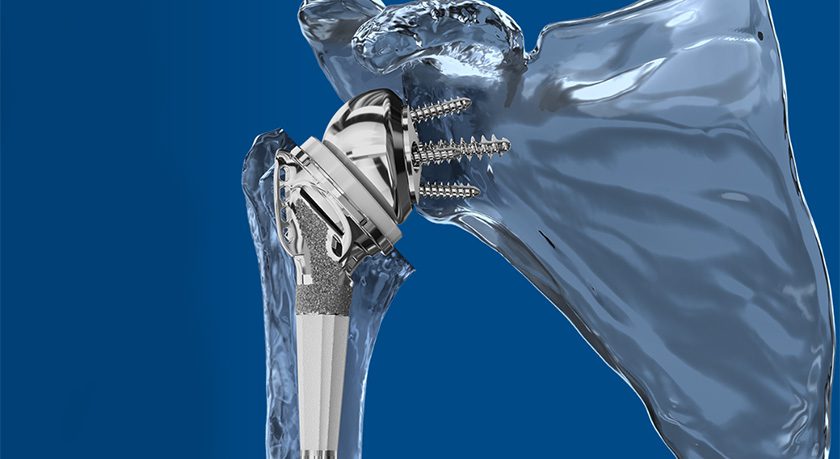
LinQ is designed to be a safe, minimally invasive option to treat SI joint dysfunction. Without drilling, a single LinQ implant with a large graft window is placed into the SI joint to promote stabilization and create an ideal environment for long-term fusion. Given the positive outcomes for patients and its excellent safety profile, PainTEQ has experienced continuous demand for its SI joint stabilization system. To date, more than 8,000 LinQ procedures have been performed in the United States.
“We are excited to work with MVP. Our ability to secure attractive debt financing from an experienced healthcare investor is a testament to the strength and potential of PainTEQ’s business”, said Sean LaNeve, CEO of PainTEQ. “This strategic funding allows us to accelerate our growth trajectory and significantly strengthens PainTEQ’s ability to improve the quality of life for an even greater number of patients living with SI joint pain.”
Source: PainTEQ
PainTEQ closed $35 million in non-dilutive growth capital backed by its intellectual property portfolio from a lending vehicle managed by MVolution Partners.
Net proceeds from this IP-backed debt financing will be used to expand the commercialization of LinQ, PainTEQ’s implantable sacroiliac (SI) joint stabilization system, as well as...
PainTEQ closed $35 million in non-dilutive growth capital backed by its intellectual property portfolio from a lending vehicle managed by MVolution Partners.
Net proceeds from this IP-backed debt financing will be used to expand the commercialization of LinQ, PainTEQ’s implantable sacroiliac (SI) joint stabilization system, as well as support research and development efforts aimed at advancing care for patients with SI joint pain and dysfunction.
LinQ is designed to be a safe, minimally invasive option to treat SI joint dysfunction. Without drilling, a single LinQ implant with a large graft window is placed into the SI joint to promote stabilization and create an ideal environment for long-term fusion. Given the positive outcomes for patients and its excellent safety profile, PainTEQ has experienced continuous demand for its SI joint stabilization system. To date, more than 8,000 LinQ procedures have been performed in the United States.
“We are excited to work with MVP. Our ability to secure attractive debt financing from an experienced healthcare investor is a testament to the strength and potential of PainTEQ’s business”, said Sean LaNeve, CEO of PainTEQ. “This strategic funding allows us to accelerate our growth trajectory and significantly strengthens PainTEQ’s ability to improve the quality of life for an even greater number of patients living with SI joint pain.”
Source: PainTEQ

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.