
 Copy to clipboard
Copy to clipboard 
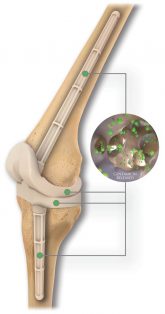
The REMEDY Stemmed Knee Spacer’s interconnected pores allow for extended antibiotic elution while improving OR efficiency vs handmade IM dowels.
OsteoRemedies commenced the full commercial launch of the REMEDY® Stemmed Knee Spacer. The device represents the first preformed knee spacer with attachable modular stems that can connect to the femoral and tibial component and is an extension of the company’s portfolio of preformed modular spacers with Gentamicin for the hip, knee, and shoulder, as well the Gentamicin + Vancomycin (GV) preformed modular spacer and bone cement for the hip.
Eric Stookey, COO, OsteoRemedies noted, “After 5 years of clinical experience with the current knee spacer design, our surgeon design team and users clearly identified the need for additional knee stem extensions for use in more complex two-stage revisions for infection. By incorporating our proprietary pre-molded and modular approach to the knee stem design, we can offer surgeons the opportunity to improve O.R. efficiency vs handmade IM dowels.”
The company’s REMEDY Spacer was the first available modular system for hip, knee, and shoulder two-stage infection revision arthroplasty. SPECTRUM® GV Bone Cement is indicated for use with the REMEDY SPECTRUM GV Hip Spacer. Other products marketed by OsteoRemedies® include the OSTEOBOOST® Resorbable Bead Kit, FLORASEAL® Microbial Sealant and UNITE® AB Bone Cement.
OsteoRemedies commenced the full commercial launch of the REMEDY® Stemmed Knee Spacer. The device represents the first preformed knee spacer with attachable modular stems that can connect to the femoral and tibial component and is an extension of the company's portfolio of preformed modular spacers with Gentamicin for the hip, knee, and...

The REMEDY Stemmed Knee Spacer’s interconnected pores allow for extended antibiotic elution while improving OR efficiency vs handmade IM dowels.
OsteoRemedies commenced the full commercial launch of the REMEDY® Stemmed Knee Spacer. The device represents the first preformed knee spacer with attachable modular stems that can connect to the femoral and tibial component and is an extension of the company’s portfolio of preformed modular spacers with Gentamicin for the hip, knee, and shoulder, as well the Gentamicin + Vancomycin (GV) preformed modular spacer and bone cement for the hip.
Eric Stookey, COO, OsteoRemedies noted, “After 5 years of clinical experience with the current knee spacer design, our surgeon design team and users clearly identified the need for additional knee stem extensions for use in more complex two-stage revisions for infection. By incorporating our proprietary pre-molded and modular approach to the knee stem design, we can offer surgeons the opportunity to improve O.R. efficiency vs handmade IM dowels.”
The company’s REMEDY Spacer was the first available modular system for hip, knee, and shoulder two-stage infection revision arthroplasty. SPECTRUM® GV Bone Cement is indicated for use with the REMEDY SPECTRUM GV Hip Spacer. Other products marketed by OsteoRemedies® include the OSTEOBOOST® Resorbable Bead Kit, FLORASEAL® Microbial Sealant and UNITE® AB Bone Cement.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







