
 Copy to clipboard
Copy to clipboard 
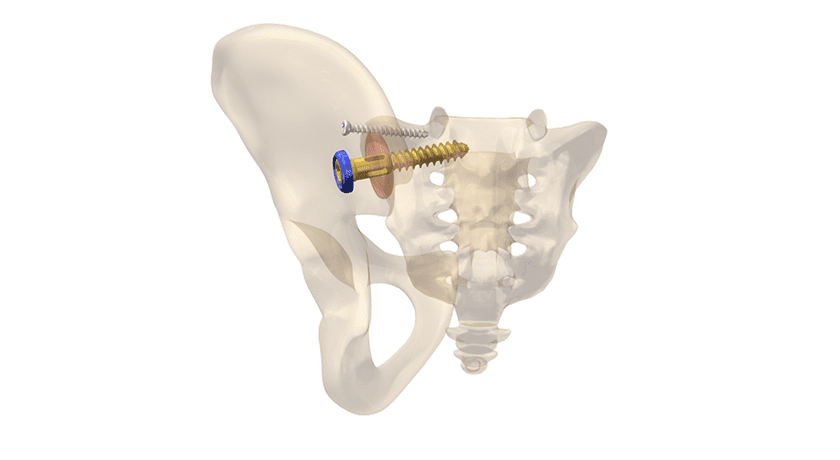
OsteoCentric Technologies was issued two new FDA 510(k) clearances. The OsteoCentric Pedicle Screw Fastener System™ will feature UnifiMI technology, and the Integrity-SI Fusion System™ now includes indications in trauma surgery.
Due to the importance of acute fixation and multi-axis stability in spine surgery, integrating the Mechanical Integration (UnifiMI) method into a pedicle system is an impactful strategic pathway following the market disruption of UnifiMI technology in the orthopedic trauma space.
Utilizing UnifiMI, the OsteoCentric Pedicle Screw Fastener System is designed to create stability between the pedicle and implant, limiting construct loosening and enhancing stability.
“The UnifiMI Pedicle Fastener will be the only system on the market leveraging Mechanical Integration technology to address implant instability at the bone-implant interface,” says Eric Brown, Founder, and CEO of the company.
The Integrity-SI Fusion System featuring UnifiMI clearance expands the System indications for use in SI trauma.
Source: OsteoCentric Technologies
OsteoCentric Technologies was issued two new FDA 510(k) clearances. The OsteoCentric Pedicle Screw Fastener System™ will feature UnifiMI technology, and the Integrity-SI Fusion System™ now includes indications in trauma surgery.
Due to the importance of acute fixation and multi-axis stability in spine surgery, integrating the Mechanical...
OsteoCentric Technologies was issued two new FDA 510(k) clearances. The OsteoCentric Pedicle Screw Fastener System™ will feature UnifiMI technology, and the Integrity-SI Fusion System™ now includes indications in trauma surgery.
Due to the importance of acute fixation and multi-axis stability in spine surgery, integrating the Mechanical Integration (UnifiMI) method into a pedicle system is an impactful strategic pathway following the market disruption of UnifiMI technology in the orthopedic trauma space.
Utilizing UnifiMI, the OsteoCentric Pedicle Screw Fastener System is designed to create stability between the pedicle and implant, limiting construct loosening and enhancing stability.
“The UnifiMI Pedicle Fastener will be the only system on the market leveraging Mechanical Integration technology to address implant instability at the bone-implant interface,” says Eric Brown, Founder, and CEO of the company.
The Integrity-SI Fusion System featuring UnifiMI clearance expands the System indications for use in SI trauma.
Source: OsteoCentric Technologies

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







