
 Copy to clipboard
Copy to clipboard 
OSSIO commenced U.S. launch of the OSSIOfiber® Trimmable Fixation Nail to fixate bone fractures, osteotomies and arthrodeses. The device leaves the patient’s bone restored with no permanent hardware left behind.
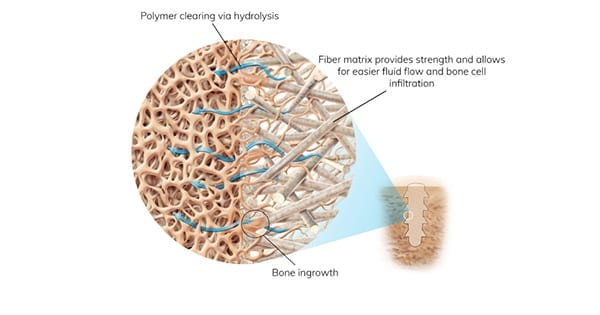
Part of the OSSIOfiber® Bone Pin Product Family, which received FDA 510(k) clearance in 2019, the OSSIOfiber Trimmable Fixation Nail includes biointegrative Trimmable Fixation Nails packaged with sterile, disposable instrumentation. The nails present an alternative to metal compression screws, combining strength for bone fixation with the ability to provide rapid bone in-growth, regeneration and replacement without adverse inflammation. The ability to trim the implants to any length enables customization to patient anatomy and adaptability for a wide range of indications including forefoot, midfoot, hindfoot and hand/wrist applications.
OSSIO’s OSSIOfiber technology is engineered from a matrix of reinforcing mineral fibers yielding strength that is 1.5 times greater than cortical bone.
Brian Verrier, CEO of OSSIO, said, “Adoption of our OSSIOfiber Bone Pin Family has been strong to date, with substantial customer utilization across the U.S. We look forward to bringing additional breakthrough technology platforms to market that utilize our proprietary OSSIOfiber technology and deliver a new standard of care in orthopedic fixation.”
OSSIO commenced U.S. launch of the OSSIOfiber® Trimmable Fixation Nail to fixate bone fractures, osteotomies and arthrodeses. The device leaves the patient’s bone restored with no permanent hardware left behind.
Part of the OSSIOfiber® Bone Pin Product Family, which received FDA 510(k) clearance in 2019, the OSSIOfiber Trimmable...
OSSIO commenced U.S. launch of the OSSIOfiber® Trimmable Fixation Nail to fixate bone fractures, osteotomies and arthrodeses. The device leaves the patient’s bone restored with no permanent hardware left behind.
Part of the OSSIOfiber® Bone Pin Product Family, which received FDA 510(k) clearance in 2019, the OSSIOfiber Trimmable Fixation Nail includes biointegrative Trimmable Fixation Nails packaged with sterile, disposable instrumentation. The nails present an alternative to metal compression screws, combining strength for bone fixation with the ability to provide rapid bone in-growth, regeneration and replacement without adverse inflammation. The ability to trim the implants to any length enables customization to patient anatomy and adaptability for a wide range of indications including forefoot, midfoot, hindfoot and hand/wrist applications.
OSSIO’s OSSIOfiber technology is engineered from a matrix of reinforcing mineral fibers yielding strength that is 1.5 times greater than cortical bone.
Brian Verrier, CEO of OSSIO, said, “Adoption of our OSSIOfiber Bone Pin Family has been strong to date, with substantial customer utilization across the U.S. We look forward to bringing additional breakthrough technology platforms to market that utilize our proprietary OSSIOfiber technology and deliver a new standard of care in orthopedic fixation.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







