
 Copy to clipboard
Copy to clipboard 
OSSIO was granted its third FDA 510(k) clearance for the OSSIOfiber® product family in the past four months.
OSSIO’s three most recent FDA clearances have been granted since December 2021:
OSSIOfiber® Suture Anchors for use in fixation of suture (soft tissue) to bone in the shoulder, foot/ankle, knee, hand/wrist and elbow. U.S. launch in 2Q22.
OSSIOfiber® Compression Staples for use in fixation of arthrodesis, osteotomies and fractures in hand or foot surgery in the presence of appropriate brace and/or immobilization. U.S. launch in 3Q22.
OSSIOfiber® 3.5mm Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot. U.S. launch in 3Q22.
With the addition of these three new FDA-cleared product platforms, OSSIO is doubling the size of its commercial product portfolio, increasing surgeon and patient access to “all-natural” treatment options and expanding the company’s total addressable market opportunity.
Previously launched OSSIOfiber implants include OSSIOfiber Compression Screws, the Trimmable Fixation Nail Family and the Hammertoe Fixation System.
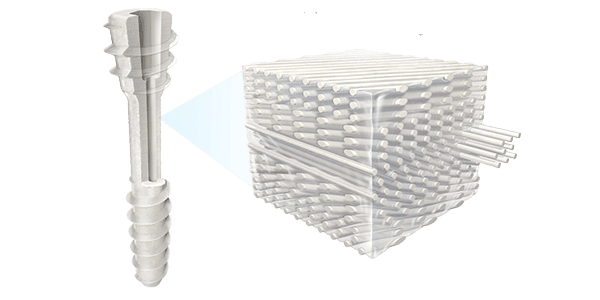
All OSSIO implants are made with OSSIOfiber Intelligent Bone Regeneration Technology, a fixation material that provides an alternative to permanent metal hardware, conventional resorbable and allograft implants, combining mechanical strength and natural bone healing in a non-permanent implant. Made from a proprietary mineral fiber matrix held together by a naturally degradable polymer, its bio-integrative material properties provide surgeons with a biologically friendly way to restore patient stability and mobility while leaving nothing permanent behind.
OSSIOfiber Intelligent Bone Regeneration Technology can address many surgical applications through the manufacturing of endless implant designs, including nails, screws, staples, anchors and plates. The company intends to pursue multiple applications in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.
Source: OSSIO
OSSIO was granted its third FDA 510(k) clearance for the OSSIOfiber® product family in the past four months.
OSSIO’s three most recent FDA clearances have been granted since December 2021:
OSSIOfiber® Suture Anchors for use in fixation of suture (soft tissue) to bone in the shoulder, foot/ankle, knee, hand/wrist and elbow. U.S. launch in...
OSSIO was granted its third FDA 510(k) clearance for the OSSIOfiber® product family in the past four months.
OSSIO’s three most recent FDA clearances have been granted since December 2021:
OSSIOfiber® Suture Anchors for use in fixation of suture (soft tissue) to bone in the shoulder, foot/ankle, knee, hand/wrist and elbow. U.S. launch in 2Q22.
OSSIOfiber® Compression Staples for use in fixation of arthrodesis, osteotomies and fractures in hand or foot surgery in the presence of appropriate brace and/or immobilization. U.S. launch in 3Q22.
OSSIOfiber® 3.5mm Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot. U.S. launch in 3Q22.
With the addition of these three new FDA-cleared product platforms, OSSIO is doubling the size of its commercial product portfolio, increasing surgeon and patient access to “all-natural” treatment options and expanding the company’s total addressable market opportunity.
Previously launched OSSIOfiber implants include OSSIOfiber Compression Screws, the Trimmable Fixation Nail Family and the Hammertoe Fixation System.
All OSSIO implants are made with OSSIOfiber Intelligent Bone Regeneration Technology, a fixation material that provides an alternative to permanent metal hardware, conventional resorbable and allograft implants, combining mechanical strength and natural bone healing in a non-permanent implant. Made from a proprietary mineral fiber matrix held together by a naturally degradable polymer, its bio-integrative material properties provide surgeons with a biologically friendly way to restore patient stability and mobility while leaving nothing permanent behind.
OSSIOfiber Intelligent Bone Regeneration Technology can address many surgical applications through the manufacturing of endless implant designs, including nails, screws, staples, anchors and plates. The company intends to pursue multiple applications in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.
Source: OSSIO

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







