
 Copy to clipboard
Copy to clipboard 
OSSIO was granted FDA 510(k) clearance to market OSSIOfiber® Hammertoe Fixation for alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts.
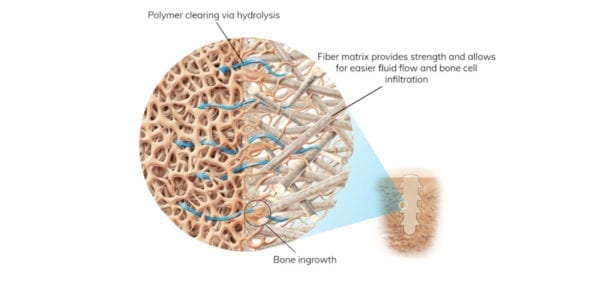
The company’s bone pin products were cleared and entered limited market launch early last year to treat common forefoot conditions where hardware removal surgeries are prevalent. OSSIOfiber Hammertoe Fixation uses the company’s OSSIOfiber Intelligent Bone Regeneration Technology, a new category of fixation material that combines mechanical strength and natural bone healing in a non-permanent implant. Bio-integrative material properties offer a biologically friendly way to restore patient stability and mobility while leaving nothing permanent behind.
In a European multi-center clinical trial examining the safety and performance of OSSIOfiber Hammertoe Fixation, 6-month results demonstrated fusion rates well above historical literature, improvements in pain and quality-of-life scores from baseline, radiographic evidence of good bio-integration with the surrounding anatomy and no evidence of adverse biological response or serious adverse events. All participating 25 patients would recommend the technology to others. Findings will support OSSIO’s CE Mark application later this year.
OSSIO plans to launch other OSSIOfiber fiber products in 2020 and will pursue applications in distal extremity, trauma, sports, reconstruction, pediatrics, and spine segments.
“With more than 500 successful hammertoe repairs conducted to date utilizing the OSSIOfiber Hammertoe Implant, along with the completion of our European multi-center study confirming the overall safety and positive performance of our proprietary technology, confidence in achieving excellent clinical outcomes and high surgeon and patient satisfaction continues to grow,” said Brian Verrier, CEO, OSSIO. “Launching the broader hammertoe offering along with several other OSSIOfiber product platforms over the next year will serve a broader orthopedic fixation market that aspires to avoid the risks, costs and trade-offs associated with permanent implants.”
OSSIO was granted FDA 510(k) clearance to market OSSIOfiber® Hammertoe Fixation for alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts.
The company's bone pin products were cleared and entered limited market launch early last year to treat common forefoot conditions where hardware removal surgeries are...
OSSIO was granted FDA 510(k) clearance to market OSSIOfiber® Hammertoe Fixation for alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts.
The company’s bone pin products were cleared and entered limited market launch early last year to treat common forefoot conditions where hardware removal surgeries are prevalent. OSSIOfiber Hammertoe Fixation uses the company’s OSSIOfiber Intelligent Bone Regeneration Technology, a new category of fixation material that combines mechanical strength and natural bone healing in a non-permanent implant. Bio-integrative material properties offer a biologically friendly way to restore patient stability and mobility while leaving nothing permanent behind.
In a European multi-center clinical trial examining the safety and performance of OSSIOfiber Hammertoe Fixation, 6-month results demonstrated fusion rates well above historical literature, improvements in pain and quality-of-life scores from baseline, radiographic evidence of good bio-integration with the surrounding anatomy and no evidence of adverse biological response or serious adverse events. All participating 25 patients would recommend the technology to others. Findings will support OSSIO’s CE Mark application later this year.
OSSIO plans to launch other OSSIOfiber fiber products in 2020 and will pursue applications in distal extremity, trauma, sports, reconstruction, pediatrics, and spine segments.
“With more than 500 successful hammertoe repairs conducted to date utilizing the OSSIOfiber Hammertoe Implant, along with the completion of our European multi-center study confirming the overall safety and positive performance of our proprietary technology, confidence in achieving excellent clinical outcomes and high surgeon and patient satisfaction continues to grow,” said Brian Verrier, CEO, OSSIO. “Launching the broader hammertoe offering along with several other OSSIOfiber product platforms over the next year will serve a broader orthopedic fixation market that aspires to avoid the risks, costs and trade-offs associated with permanent implants.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







