
 Copy to clipboard
Copy to clipboard 
Umbra Applied Technologies, parent company of Ossifix Technologies, has begun fulfilling purchase orders and contracts for orthopedic distributors and clients in the U.S.
Ossifix engineers and manufactures 100% allograft bone implants for small bone/extremities (trauma) and sports medicine markets that also have application in craniofacial, spine and oral surgery procedures. No regulatory approval is required for implantable fixation engineered from allograft bone. As a result, barriers to entry into the market for Ossifix Technologies implants are minimal.
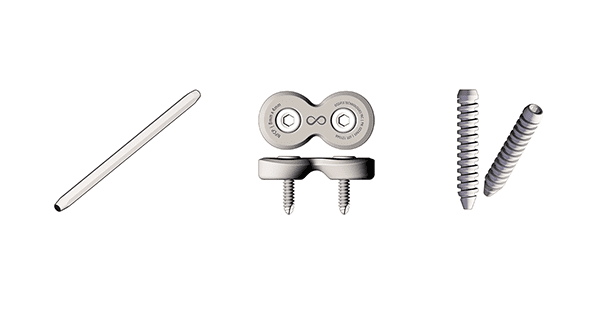
Ossifix Orthopedics products are manufactured from high-density allograft cortical bone. The company’s manufacturing process and patent pending designs yield an engineered cortical bone implant that provides secure fixation and virtually eliminates allergic or autoimmune reactions associated with metal or plastic/polymer implants. All Ossifix Orthopedics pin, screw and plate systems comprise single-use surgical kits with all necessary instruments for implantation. Instrumentation is constructed of an eco-friendly, biodegradable polymer.
Ossifix CEO, Mark Estrada, said, “These initial sales are the foundation upon which we anticipate building exponential growth in sales. We will begin rolling out the Jarvis bone pin immediately followed by additional products in the coming weeks.”
Ossifix is expected to post revenues within 1Q22.
The Ossifix Orthopedics line of products (sampled above) will compete in the resorbable implant market, in three distinct surgical segments: orthopedic, dental and general. Ossifix products are designed to be used in hospitals, ambulatory surgical centers and specialty clinics. Distribution is scheduled throughout North America, and globally in Latin America, Europe, Asia Pacific, as well as the Middle East and Africa.
Umbra Applied Technologies, parent company of Ossifix Technologies, has begun fulfilling purchase orders and contracts for orthopedic distributors and clients in the U.S.
Ossifix engineers and manufactures 100% allograft bone implants for small bone/extremities (trauma) and sports medicine markets that also have application in craniofacial,...
Umbra Applied Technologies, parent company of Ossifix Technologies, has begun fulfilling purchase orders and contracts for orthopedic distributors and clients in the U.S.
Ossifix engineers and manufactures 100% allograft bone implants for small bone/extremities (trauma) and sports medicine markets that also have application in craniofacial, spine and oral surgery procedures. No regulatory approval is required for implantable fixation engineered from allograft bone. As a result, barriers to entry into the market for Ossifix Technologies implants are minimal.
Ossifix Orthopedics products are manufactured from high-density allograft cortical bone. The company’s manufacturing process and patent pending designs yield an engineered cortical bone implant that provides secure fixation and virtually eliminates allergic or autoimmune reactions associated with metal or plastic/polymer implants. All Ossifix Orthopedics pin, screw and plate systems comprise single-use surgical kits with all necessary instruments for implantation. Instrumentation is constructed of an eco-friendly, biodegradable polymer.
Ossifix CEO, Mark Estrada, said, “These initial sales are the foundation upon which we anticipate building exponential growth in sales. We will begin rolling out the Jarvis bone pin immediately followed by additional products in the coming weeks.”
Ossifix is expected to post revenues within 1Q22.
The Ossifix Orthopedics line of products (sampled above) will compete in the resorbable implant market, in three distinct surgical segments: orthopedic, dental and general. Ossifix products are designed to be used in hospitals, ambulatory surgical centers and specialty clinics. Distribution is scheduled throughout North America, and globally in Latin America, Europe, Asia Pacific, as well as the Middle East and Africa.
Source: Umbra Applied Technologies Group, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







