
 Copy to clipboard
Copy to clipboard 
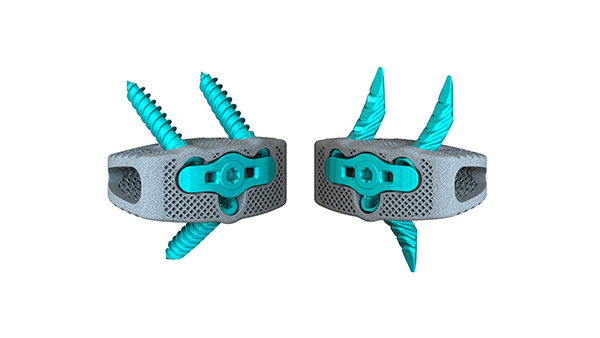
Osseus announced FDA 510(k) clearance and launch of the Pisces™-SA Standalone ALIF Interbody System. Pisces-SA can be used with both bone screws and alternative fixation bone anchors allowing for increased intraoperative flexibility.
Biomechanical testing has indicated that Pisces-SA anchors provide better expulsion resistance than the competition and perform comparably with traditional screw-based standalone ALIF constructs in stabilizing injured spinal segments. Pisces-SA is reportedly the first of its kind to provide this level of expulsion resistance and segmental stabilization using an alternative fixation method.
Pisces-SA anchors allow for a streamlined, direct anterior approach which facilitates minimal access and improved operational efficiency.
The platform integrates a highly-porous 3D-printed interbody with anatomical morphology designed for full osseointegration with streamlined instrumentation to facilitate a minimally invasive approach.
Source: Osseus Fusion Systems
Osseus announced FDA 510(k) clearance and launch of the Pisces™-SA Standalone ALIF Interbody System. Pisces-SA can be used with both bone screws and alternative fixation bone anchors allowing for increased intraoperative flexibility.
Biomechanical testing has indicated that Pisces-SA anchors provide better expulsion resistance than the...
Osseus announced FDA 510(k) clearance and launch of the Pisces™-SA Standalone ALIF Interbody System. Pisces-SA can be used with both bone screws and alternative fixation bone anchors allowing for increased intraoperative flexibility.
Biomechanical testing has indicated that Pisces-SA anchors provide better expulsion resistance than the competition and perform comparably with traditional screw-based standalone ALIF constructs in stabilizing injured spinal segments. Pisces-SA is reportedly the first of its kind to provide this level of expulsion resistance and segmental stabilization using an alternative fixation method.
Pisces-SA anchors allow for a streamlined, direct anterior approach which facilitates minimal access and improved operational efficiency.
The platform integrates a highly-porous 3D-printed interbody with anatomical morphology designed for full osseointegration with streamlined instrumentation to facilitate a minimally invasive approach.
Source: Osseus Fusion Systems

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







