Copy to clipboard
Copy to clipboard 
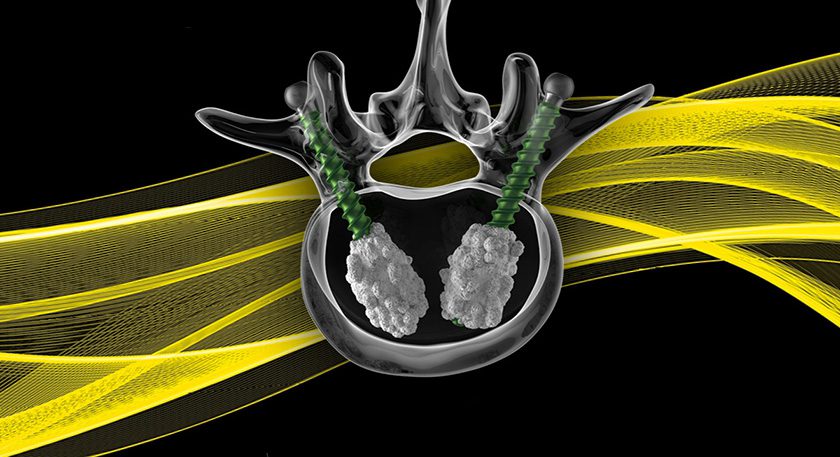
OssDsign received review board approval to establish PROPEL, a multi-center prospective spinal fusion registry, to study real-world data from patients who have been treated with OssDsign Catalyst.
OssDsign Catalyst received FDA clearance in 2020 and was launched in the U.S. in 2021. During 2022, clinics will gradually be enrolled to the registry, with the objective to evaluate the use and outcome of OssDsign Catalyst in a real-world clinical practice. The primary endpoint of the study will be measuring the rate of spinal fusion, using computer tomography or radiography, 12 months postoperatively. Additionally, patients’ quality of life and neurological function, as well as the clinical safety profile of the spinal implant will be recorded.
Concurrently, the company’s TOP FUSION clinical study, evaluating the long-term safety and efficacy of OssDsign Catalyst in patients, is continuing to enroll subjects in Hungary.
“The registry represents one of the core pillars in our long-term strategy and I am pleased that we continue to deliver on the strategic plan. Robust clinical data is key to establish our products on the market and PROPEL allows us to collect clinical data on large numbers of patients, receiving care in diverse clinical settings, among U.S. surgeons,” said Morten Henneveld, CEO of OssDsign.
Source: OssDsign
OssDsign received review board approval to establish PROPEL, a multi-center prospective spinal fusion registry, to study real-world data from patients who have been treated with OssDsign Catalyst.
OssDsign Catalyst received FDA clearance in 2020 and was launched in the U.S. in 2021. During 2022, clinics will gradually be enrolled to the...
OssDsign received review board approval to establish PROPEL, a multi-center prospective spinal fusion registry, to study real-world data from patients who have been treated with OssDsign Catalyst.
OssDsign Catalyst received FDA clearance in 2020 and was launched in the U.S. in 2021. During 2022, clinics will gradually be enrolled to the registry, with the objective to evaluate the use and outcome of OssDsign Catalyst in a real-world clinical practice. The primary endpoint of the study will be measuring the rate of spinal fusion, using computer tomography or radiography, 12 months postoperatively. Additionally, patients’ quality of life and neurological function, as well as the clinical safety profile of the spinal implant will be recorded.
Concurrently, the company’s TOP FUSION clinical study, evaluating the long-term safety and efficacy of OssDsign Catalyst in patients, is continuing to enroll subjects in Hungary.
“The registry represents one of the core pillars in our long-term strategy and I am pleased that we continue to deliver on the strategic plan. Robust clinical data is key to establish our products on the market and PROPEL allows us to collect clinical data on large numbers of patients, receiving care in diverse clinical settings, among U.S. surgeons,” said Morten Henneveld, CEO of OssDsign.
Source: OssDsign

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.