Copy to clipboard
Copy to clipboard 
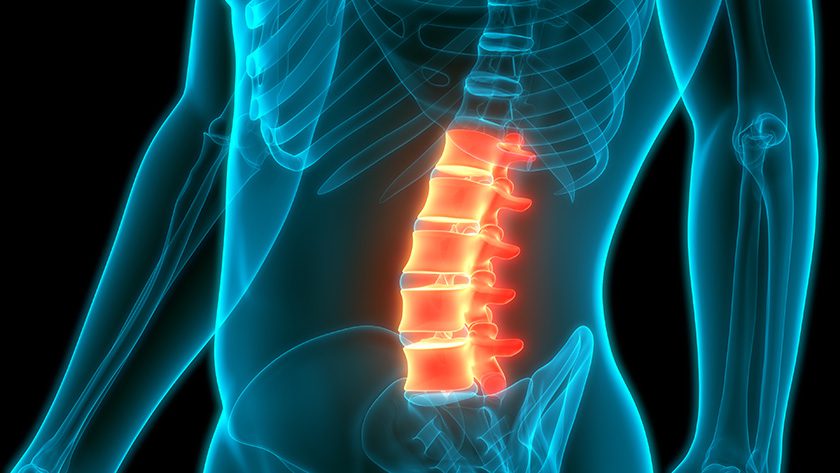
OssDsign published one-year results from the first 108 patients in its prospective, multi-center, spinal fusion registry PROPEL. The results show an outstanding fusion rate of 88.4% in the real-world setting in a highly complex patient cohort, demonstrating that OssDsign Catalyst nanosynthetic bone graft shows strong performance even in challenging patient populations with high BMI, previous failed fusion surgeries, smokers, multi-level procedures and several comorbidities.
The results are based on 108 patients and the studied population is considered a highly complex patient cohort with the following profile:
- Average BMI of 31.9 (obese)
- 93.6% of patients had at least one comorbidity with 48% having three or more comorbidities
- 50% had previous spine fusion surgery performed
- 48.0% were active or previous smokers
- 20.3% of all procedures involved three or more levels of the spine
At the 12-month follow-up, all patients showed improvement in each health status category assessed, with no unexpected device-related adverse events and a successful fusion rate of 88.4%, and between 84.7% and 93.4% in high-risk subgroups.
“These results are a crucial building block in OssDsign’s clinical strategy, showing OssDsign Catalyst to be a robust, differentiated nanosynthetic bone graft that performs as intended regardless of patient profile. Previously, our TOP FUSION first-in-human study reported a 93% fusion rate and improvements in all health status categories at the 12-month follow-up. Although we anticipated strong real-world performance, the remarkable consistency with our clinical trial results exceeds our expectations.
“These results prove that OssDsign Catalyst completely transforms expectations for fusion surgery outcomes, offering comfort and confidence that all patients, whether complex or simple, can return to an active life,” says Morten Henneveld, CEO of OssDsign.
Source: OssDsign
OssDsign published one-year results from the first 108 patients in its prospective, multi-center, spinal fusion registry PROPEL. The results show an outstanding fusion rate of 88.4% in the real-world setting in a highly complex patient cohort, demonstrating that OssDsign Catalyst nanosynthetic bone graft shows strong performance even in...
OssDsign published one-year results from the first 108 patients in its prospective, multi-center, spinal fusion registry PROPEL. The results show an outstanding fusion rate of 88.4% in the real-world setting in a highly complex patient cohort, demonstrating that OssDsign Catalyst nanosynthetic bone graft shows strong performance even in challenging patient populations with high BMI, previous failed fusion surgeries, smokers, multi-level procedures and several comorbidities.
The results are based on 108 patients and the studied population is considered a highly complex patient cohort with the following profile:
- Average BMI of 31.9 (obese)
- 93.6% of patients had at least one comorbidity with 48% having three or more comorbidities
- 50% had previous spine fusion surgery performed
- 48.0% were active or previous smokers
- 20.3% of all procedures involved three or more levels of the spine
At the 12-month follow-up, all patients showed improvement in each health status category assessed, with no unexpected device-related adverse events and a successful fusion rate of 88.4%, and between 84.7% and 93.4% in high-risk subgroups.
“These results are a crucial building block in OssDsign’s clinical strategy, showing OssDsign Catalyst to be a robust, differentiated nanosynthetic bone graft that performs as intended regardless of patient profile. Previously, our TOP FUSION first-in-human study reported a 93% fusion rate and improvements in all health status categories at the 12-month follow-up. Although we anticipated strong real-world performance, the remarkable consistency with our clinical trial results exceeds our expectations.
“These results prove that OssDsign Catalyst completely transforms expectations for fusion surgery outcomes, offering comfort and confidence that all patients, whether complex or simple, can return to an active life,” says Morten Henneveld, CEO of OssDsign.
Source: OssDsign

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.