
 Copy to clipboard
Copy to clipboard 
OssDsign completed its first sales of OssDsign Cranial PSI in Japan. The launch marks a strategic milestone, following a period of postponed commercial activities due to the Covid-19 pandemic.
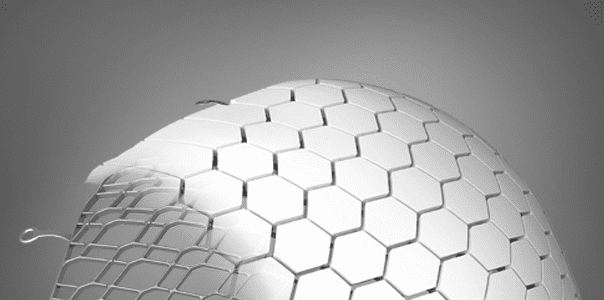
OssDsign Cranial PSI is a patient-specific cranial implant made from 3D-printed medical-grade titanium covered by a regenerative calcium phosphate composition. While the titanium skeleton reinforces the implant and resists physical and mechanical stress, the calcium phosphate composition provides healing and regenerative properties, allowing regrowth of the patient’s own bone. Over time, the calcium phosphate composition degrades and is replaced with bone, leaving the patient with a well-integrated implant, potentially lasting a lifetime.
The product is commercially available in more than ten markets worldwide through OssDsign’s direct salesforce as well as distributors and independent sales agents.
The most recent clinical data from long-term follow-up of the OssDsign Cranial PSI, based on 1,480 implants across 214 hospitals worldwide, showed that the frequency of infections leading to implant removal was 1.6% after a median follow-up time of 22 months. This positive outcome exceeded what has been observed in previous follow-ups, highlighting the performance of OssDsign Cranial PSI.
“Japan constitutes an important strategic market to our implant technology. Whilst we foresee a gradual ramp-up in the market over the coming quarters and years, I am pleased that our continued perseverance during the pandemic has resulted in the first sales and a formal launch. We will now accelerate our commercial efforts towards more Japanese hospitals,” says Morten Henneveld, CEO of OssDsign.
Source: OssDsign
OssDsign completed its first sales of OssDsign Cranial PSI in Japan. The launch marks a strategic milestone, following a period of postponed commercial activities due to the Covid-19 pandemic.
OssDsign Cranial PSI is a patient-specific cranial implant made from 3D-printed medical-grade titanium covered by a regenerative calcium phosphate...
OssDsign completed its first sales of OssDsign Cranial PSI in Japan. The launch marks a strategic milestone, following a period of postponed commercial activities due to the Covid-19 pandemic.
OssDsign Cranial PSI is a patient-specific cranial implant made from 3D-printed medical-grade titanium covered by a regenerative calcium phosphate composition. While the titanium skeleton reinforces the implant and resists physical and mechanical stress, the calcium phosphate composition provides healing and regenerative properties, allowing regrowth of the patient’s own bone. Over time, the calcium phosphate composition degrades and is replaced with bone, leaving the patient with a well-integrated implant, potentially lasting a lifetime.
The product is commercially available in more than ten markets worldwide through OssDsign’s direct salesforce as well as distributors and independent sales agents.
The most recent clinical data from long-term follow-up of the OssDsign Cranial PSI, based on 1,480 implants across 214 hospitals worldwide, showed that the frequency of infections leading to implant removal was 1.6% after a median follow-up time of 22 months. This positive outcome exceeded what has been observed in previous follow-ups, highlighting the performance of OssDsign Cranial PSI.
“Japan constitutes an important strategic market to our implant technology. Whilst we foresee a gradual ramp-up in the market over the coming quarters and years, I am pleased that our continued perseverance during the pandemic has resulted in the first sales and a formal launch. We will now accelerate our commercial efforts towards more Japanese hospitals,” says Morten Henneveld, CEO of OssDsign.
Source: OssDsign

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







