
 Copy to clipboard
Copy to clipboard 
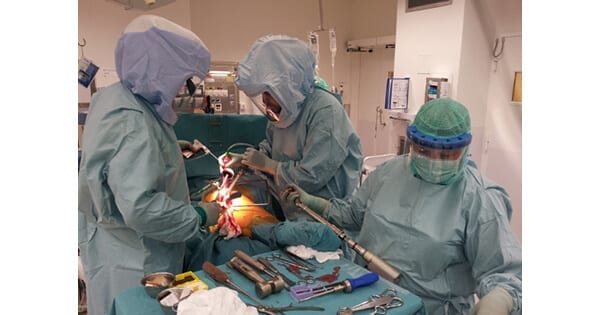
Ortoma has agreement on a non-binding term sheet with a major global orthopedics implant and MedTech manufacturer for exclusive distribution rights of the Ortoma Treatment Solution for hip surgery in Japan. The next step in the process is to sign a binding definitive agreement.
With the term sheet, the parties have a preliminary understanding of the principal commercial terms for the distribution rights of Ortoma Treatment Solution (OTS) in Japan. The term sheet covers exclusive distribution rights for OTS for hip replacement surgery including the products OTS Hip Plan and OTS Hip Guide for an initial term of 7 years. The potential income for Ortoma may, subject to a definitive agreement, be a combination of fixed fees and ongoing variable fees but cannot be quantified at this stage.
The agreement on the term sheet triggers filing of a regulatory application for marketing approval in Japan. The application can be approved within 4-6 months after filing the application. The underlying documentation for filing the regulatory application has already been prepared by Ortoma, who will bear the cost for the application in case a definitive agreement is not reached.
Ortoma’s objective is to sign the binding definitive agreement before the regulatory application is approved.
Source: Ortoma AB
Ortoma has agreement on a non-binding term sheet with a major global orthopedics implant and MedTech manufacturer for exclusive distribution rights of the Ortoma Treatment Solution for hip surgery in Japan. The next step in the process is to sign a binding definitive agreement.
With the term sheet, the parties have a preliminary understanding...
Ortoma has agreement on a non-binding term sheet with a major global orthopedics implant and MedTech manufacturer for exclusive distribution rights of the Ortoma Treatment Solution for hip surgery in Japan. The next step in the process is to sign a binding definitive agreement.
With the term sheet, the parties have a preliminary understanding of the principal commercial terms for the distribution rights of Ortoma Treatment Solution (OTS) in Japan. The term sheet covers exclusive distribution rights for OTS for hip replacement surgery including the products OTS Hip Plan and OTS Hip Guide for an initial term of 7 years. The potential income for Ortoma may, subject to a definitive agreement, be a combination of fixed fees and ongoing variable fees but cannot be quantified at this stage.
The agreement on the term sheet triggers filing of a regulatory application for marketing approval in Japan. The application can be approved within 4-6 months after filing the application. The underlying documentation for filing the regulatory application has already been prepared by Ortoma, who will bear the cost for the application in case a definitive agreement is not reached.
Ortoma’s objective is to sign the binding definitive agreement before the regulatory application is approved.
Source: Ortoma AB

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







