
 Copy to clipboard
Copy to clipboard 
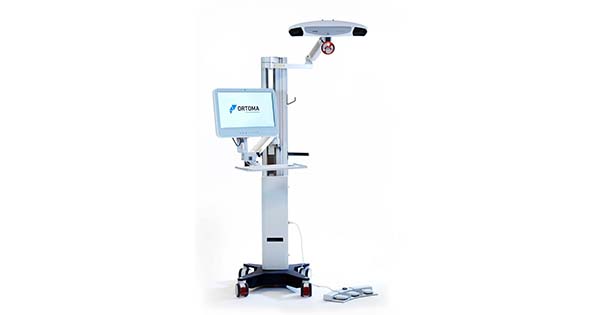
Ortoma obtained certification in Japan for its OTS Hip Plan module for pre-operative planning of hip replacement surgery. The marketing approval is an important step for launching OTS Hip, including modules for pre-operative planning and navigation of orthopedic hip implants, in Japan. The OTS platform is a surgical system that is assisted by Artificial Intelligence (AI) for improved accuracy, efficiency and surgical outcome.
Ortoma has a strategy to offer OTS Hip Plan for pre-operative planning in a stand-alone version as well as bundled with OTS Hip Guide for computer-assisted navigation. Therefore, separate regulatory applications for OTS Hip Plan and OTS Hip Guide are filed in Japan. The regulatory application for OTS Hip Plan has now been approved, and the stand-alone version of the product will be launched for clinical use in Japan. The JP regulatory application for OTS Hip Guide is pending examination. The bundle of OTS Hip plan and OTS Hip Guide will be launched in Japan when the regulatory application for OTS Hip Guide is approved.
“With the certification of OTS Hip Plan in Japan, we are ready to take the next step and introduce the product in Japan together with a strategic partner,” said Linus Byström, CEO.
In addition to the certification for marketing of OTS Hip Plan in Japan, the OTS platform includes CE marked products for hip replacement surgery and spine surgery. OTS for hip replacement surgery is also FDA 510(k) cleared for marketing in the U.S. An FDA 510(k) application for OTS Spine Plan is pending, and a regulatory application for OTS Hip Guide is pending in Japan.
Source: Ortoma
Ortoma obtained certification in Japan for its OTS Hip Plan module for pre-operative planning of hip replacement surgery. The marketing approval is an important step for launching OTS Hip, including modules for pre-operative planning and navigation of orthopedic hip implants, in Japan. The OTS platform is a surgical system that is assisted by...
Ortoma obtained certification in Japan for its OTS Hip Plan module for pre-operative planning of hip replacement surgery. The marketing approval is an important step for launching OTS Hip, including modules for pre-operative planning and navigation of orthopedic hip implants, in Japan. The OTS platform is a surgical system that is assisted by Artificial Intelligence (AI) for improved accuracy, efficiency and surgical outcome.
Ortoma has a strategy to offer OTS Hip Plan for pre-operative planning in a stand-alone version as well as bundled with OTS Hip Guide for computer-assisted navigation. Therefore, separate regulatory applications for OTS Hip Plan and OTS Hip Guide are filed in Japan. The regulatory application for OTS Hip Plan has now been approved, and the stand-alone version of the product will be launched for clinical use in Japan. The JP regulatory application for OTS Hip Guide is pending examination. The bundle of OTS Hip plan and OTS Hip Guide will be launched in Japan when the regulatory application for OTS Hip Guide is approved.
“With the certification of OTS Hip Plan in Japan, we are ready to take the next step and introduce the product in Japan together with a strategic partner,” said Linus Byström, CEO.
In addition to the certification for marketing of OTS Hip Plan in Japan, the OTS platform includes CE marked products for hip replacement surgery and spine surgery. OTS for hip replacement surgery is also FDA 510(k) cleared for marketing in the U.S. An FDA 510(k) application for OTS Spine Plan is pending, and a regulatory application for OTS Hip Guide is pending in Japan.
Source: Ortoma

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







