
 Copy to clipboard
Copy to clipboard 
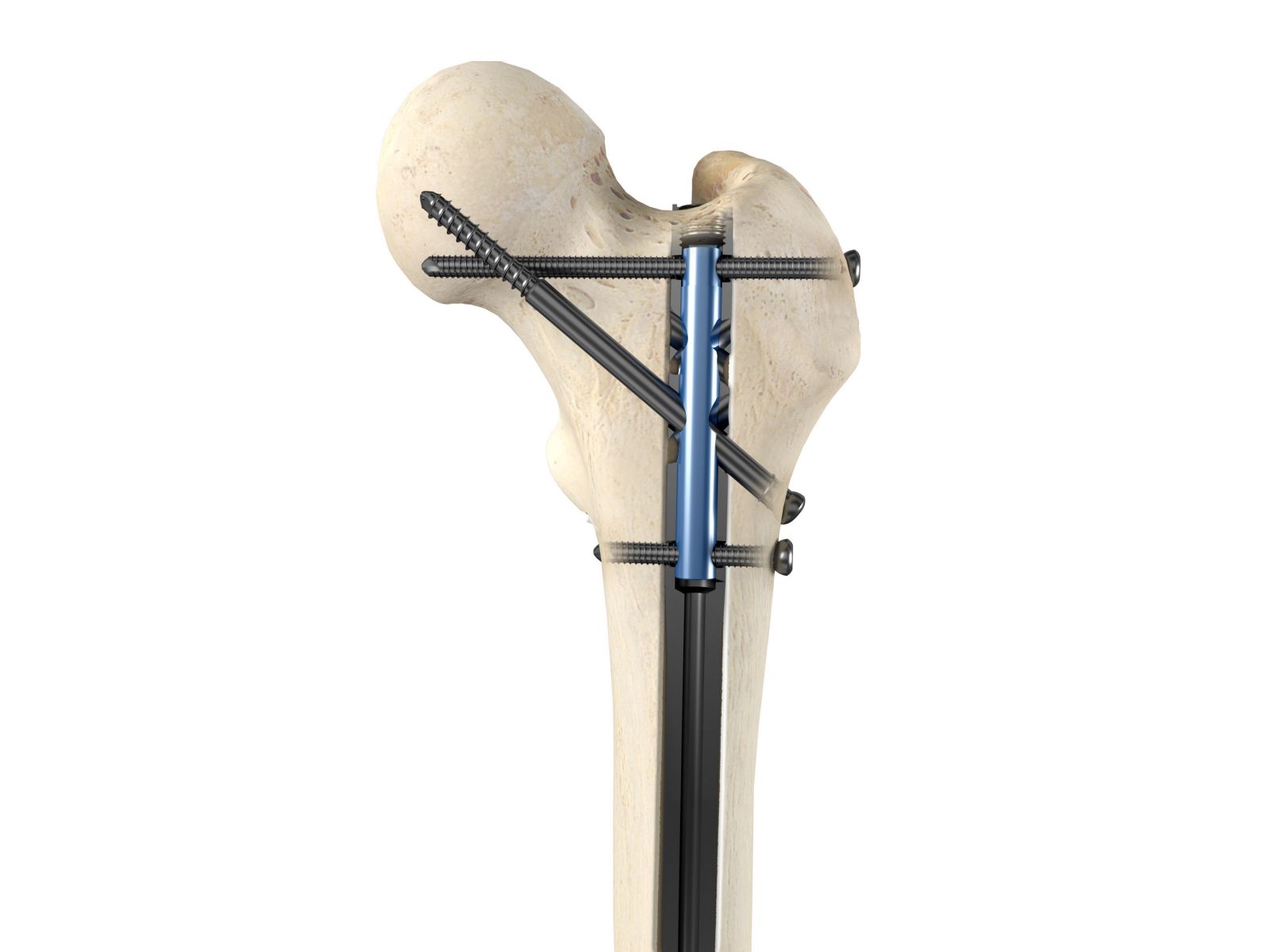
OrthoXel was granted approval under the CE Mark for the Apex Tibial Nailing System, which is now cleared for marketing the U.S. and Europe.
The universal nail can be implanted via an antegrade or retrograde approach, and features micromotion locking to stimulate the formation of bone callus. Additional locking options include recon and rigid interlocking for unstable proximal femoral fractures. A locking encap can simultaneously lock multiple screws for additional stability.
With regulatory clearances in hand, OrthoXel will now focus on clinical case studies in support of 2019 launch for Apex Femoral and Apex Tibial nails.
Source: OrthoXel
OrthoXel was granted approval under the CE Mark for the Apex Tibial Nailing System, which is now cleared for marketing the U.S. and Europe.
The universal nail can be implanted via an antegrade or retrograde approach, and features micromotion locking to stimulate the formation of bone callus. Additional locking options include recon and rigid...
OrthoXel was granted approval under the CE Mark for the Apex Tibial Nailing System, which is now cleared for marketing the U.S. and Europe.
The universal nail can be implanted via an antegrade or retrograde approach, and features micromotion locking to stimulate the formation of bone callus. Additional locking options include recon and rigid interlocking for unstable proximal femoral fractures. A locking encap can simultaneously lock multiple screws for additional stability.
With regulatory clearances in hand, OrthoXel will now focus on clinical case studies in support of 2019 launch for Apex Femoral and Apex Tibial nails.
Source: OrthoXel


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







