
 Copy to clipboard
Copy to clipboard 
Following its receipt of FDA 510(k) clearance and CE Mark approval as announced in January 2018, OrthoXel’s Apex Tibial Nail has now been implanted in the first clinical trial patient.
Apex uses controlled micromotion to stimulate callus formation in pursuit of reduced healing time. The nail offers a large range of locking options (including standard cross-locking, rigid fixation with multiple proximal screw clamping and dynamization locking with built-in torsional stability), and its design requires no changes to established reamed insertion techniques. A reusable kit allows fast implantation in any locking mode.
The company seeks partners to replicate or initiate a similar clinical trial in the U.S.
Source: OrthoXel
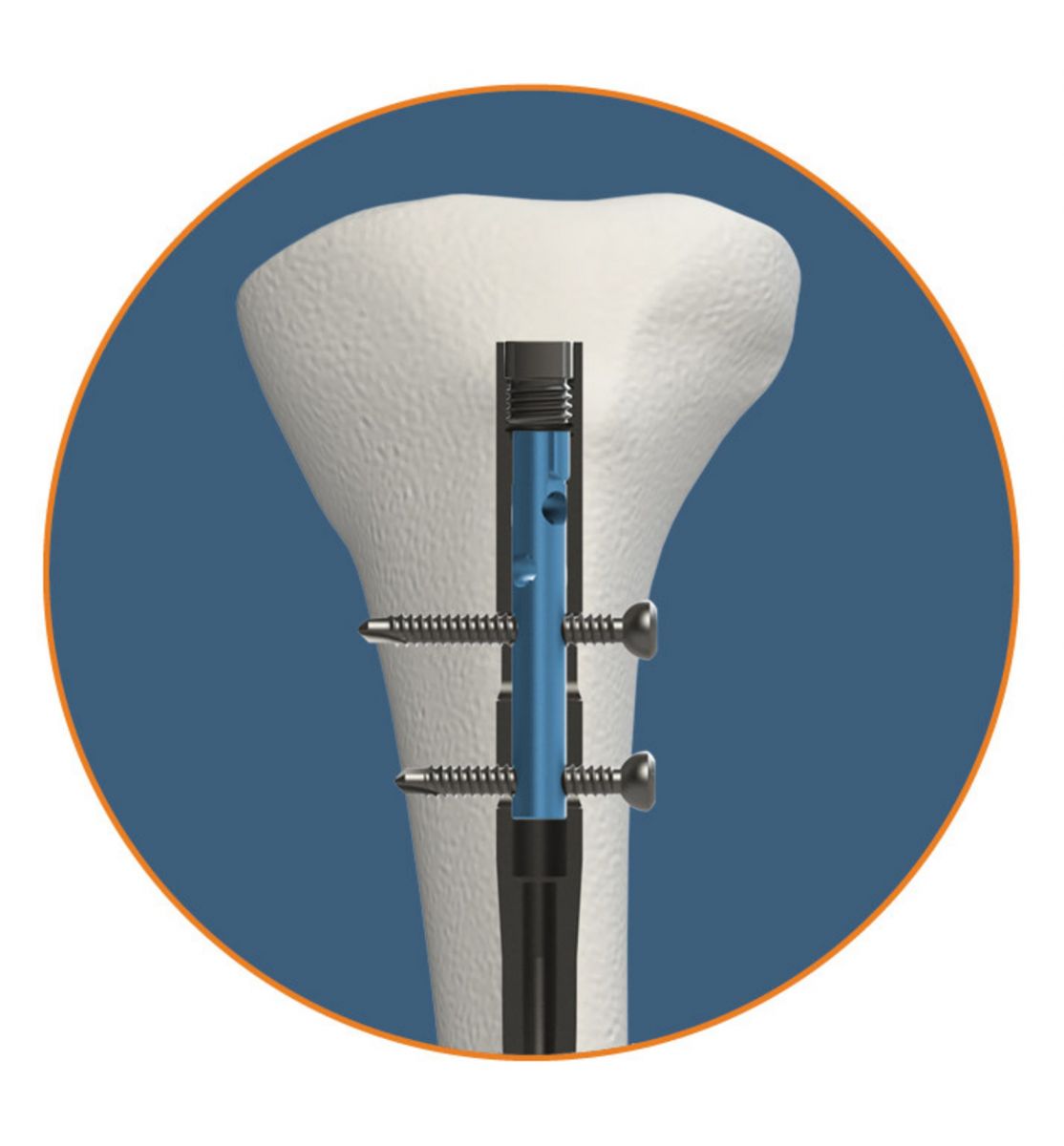
Image courtesy of OrthoXel
Following its receipt of FDA 510(k) clearance and CE Mark approval as announced in January 2018, OrthoXel's Apex Tibial Nail has now been implanted in the first clinical trial patient.
Apex uses controlled micromotion to stimulate callus formation in pursuit of reduced healing time. The nail offers a large range of locking options...
Following its receipt of FDA 510(k) clearance and CE Mark approval as announced in January 2018, OrthoXel’s Apex Tibial Nail has now been implanted in the first clinical trial patient.
Apex uses controlled micromotion to stimulate callus formation in pursuit of reduced healing time. The nail offers a large range of locking options (including standard cross-locking, rigid fixation with multiple proximal screw clamping and dynamization locking with built-in torsional stability), and its design requires no changes to established reamed insertion techniques. A reusable kit allows fast implantation in any locking mode.
The company seeks partners to replicate or initiate a similar clinical trial in the U.S.
Source: OrthoXel

Image courtesy of OrthoXel

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







