
 Copy to clipboard
Copy to clipboard 
OrthoXel received FDA 510(k) clearance and approval under the CE Mark for the Apex Tibial Nailing system.
The device reportedly offers the largest range of locking options of any intramedullary nail, and features a micromotion locking mode to support controlled axial movement with torsional stability for optimal callus formation. The design requires no changes to established evidence-based reamed insertion techniques.
Apex also provides additional locking modes such as standard cross-locking, rigid fixation with multiple proximal screw clamping and dynamization locking with built-in torsional stability.
Source: OrthoXel DAC
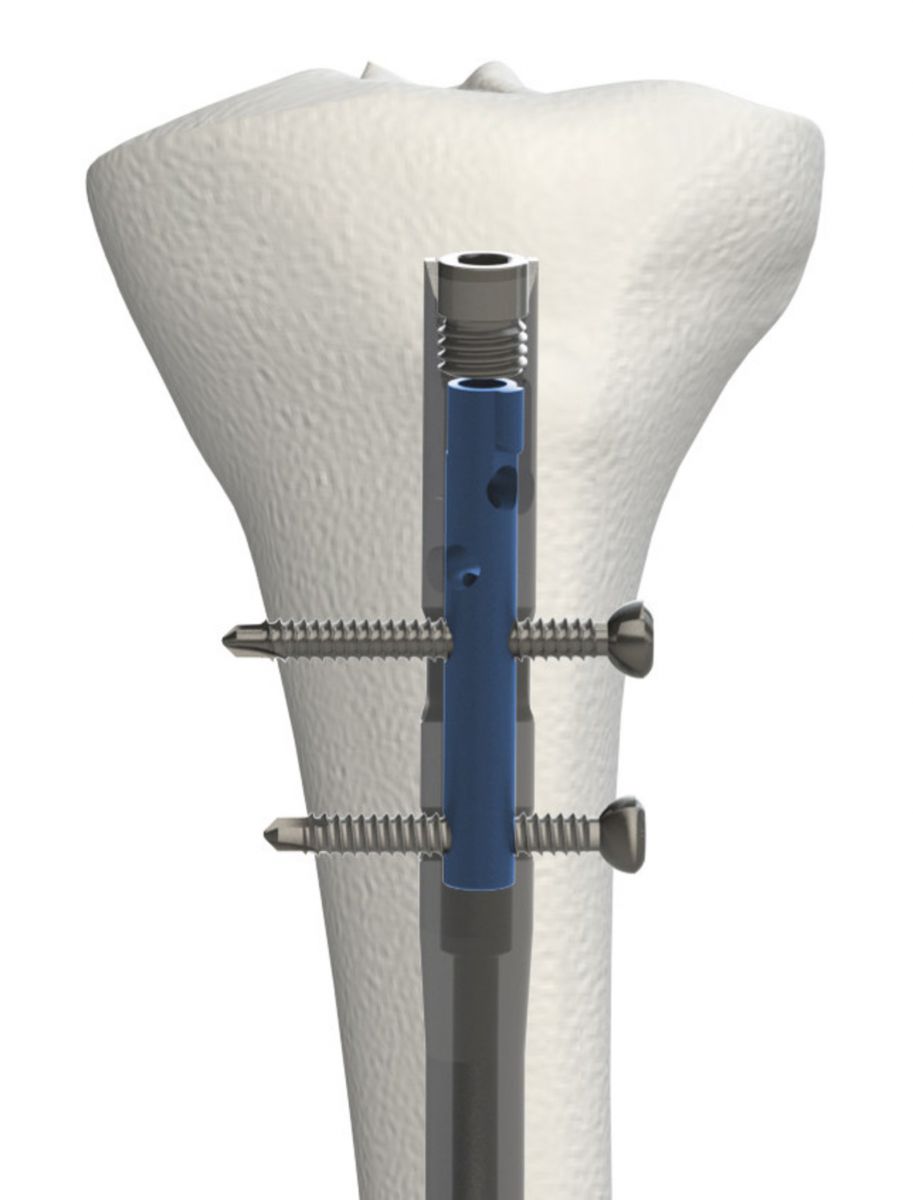
Image courtesy of OrthoXel
OrthoXel received FDA 510(k) clearance and approval under the CE Mark for the Apex Tibial Nailing system.
The device reportedly offers the largest range of locking options of any intramedullary nail, and features a micromotion locking mode to support controlled axial movement with torsional stability for optimal callus formation. The design...
OrthoXel received FDA 510(k) clearance and approval under the CE Mark for the Apex Tibial Nailing system.
The device reportedly offers the largest range of locking options of any intramedullary nail, and features a micromotion locking mode to support controlled axial movement with torsional stability for optimal callus formation. The design requires no changes to established evidence-based reamed insertion techniques.
Apex also provides additional locking modes such as standard cross-locking, rigid fixation with multiple proximal screw clamping and dynamization locking with built-in torsional stability.
Source: OrthoXel DAC

Image courtesy of OrthoXel

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







