
 Copy to clipboard
Copy to clipboard 
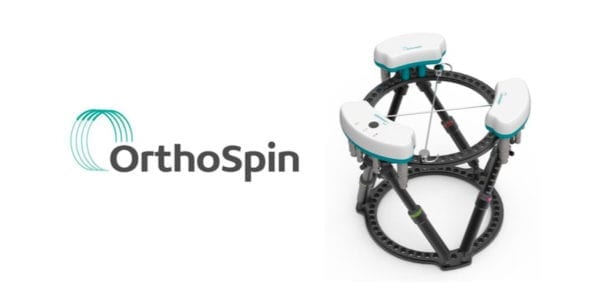
OrthoSpin received FDA 510(k) clearance to market its smart robotic external fixation system for orthopedic treatments.
The system addresses patient compliance and lack of real-time feedback for the physician. The robotic struts, which make automatic programmed adjustments, eliminate the need for manual involvement and improve patient compliance. The system monitors biomechanical parameters, and in the future will be able to automatically send real-time updates to physicians to track patient progress. This allows fine-tuning of the treatment regimen, including a personalized treatment plan for each patient.
The device was developed with investment support led by Johnson & Johnson Innovation – JJDC.
Source: OrthoSpin
OrthoSpin received FDA 510(k) clearance to market its smart robotic external fixation system for orthopedic treatments.
The system addresses patient compliance and lack of real-time feedback for the physician. The robotic struts, which make automatic programmed adjustments, eliminate the need for manual involvement and improve patient...
OrthoSpin received FDA 510(k) clearance to market its smart robotic external fixation system for orthopedic treatments.
The system addresses patient compliance and lack of real-time feedback for the physician. The robotic struts, which make automatic programmed adjustments, eliminate the need for manual involvement and improve patient compliance. The system monitors biomechanical parameters, and in the future will be able to automatically send real-time updates to physicians to track patient progress. This allows fine-tuning of the treatment regimen, including a personalized treatment plan for each patient.
The device was developed with investment support led by Johnson & Johnson Innovation – JJDC.
Source: OrthoSpin

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







