
 Copy to clipboard
Copy to clipboard 
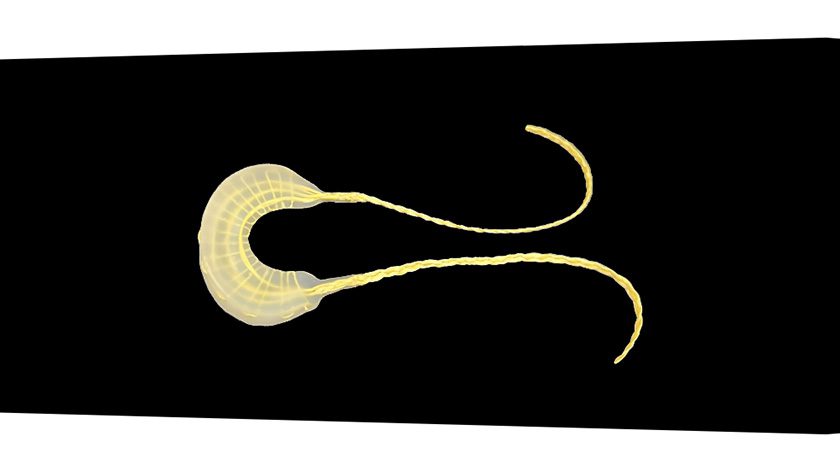
OrthoPreserve was granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from FDA for Defender, a meniscus replacement implant.
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following a meniscus surgery. Prior to January 2025, orthopedic devices were not part of the TAP program, and OrthoPreserve’s device is believed to be the first orthopedic device to win TAP enrollment.
OrthoPreserve seeks to launch their pilot clinical trial in 2026, while aiming for potential FDA clearance by 2029.
The implant potentially offers an alternative to partial meniscectomy.
Brendan Baggot, Vice President of Regulatory Affairs for OrthoPreserve, said, “TAP enrollment affords enhanced guidance by agency-selected advisors on a broad range of subjects that device startups struggle with, including market adoption and insurance coverage.”
“This Breakthrough Device designation from FDA is a major milestone for OrthoPreserve and validates the longstanding unmet medical need that our meniscus implant is designed to address,” said Jonathan Schwartz, inventor, co-founder and CEO. “The anatomical design of the implant restores the normal stabilization and cushioning functions of the meniscus to relieve symptoms and preserve knee joint health.”
Source: OrthoPreserve, Inc.
OrthoPreserve was granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from FDA for Defender, a meniscus replacement implant.
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following a meniscus...
OrthoPreserve was granted both a Breakthrough Device Designation and Total Product Life Cycle Advisory Program (TAP) enrollment from FDA for Defender, a meniscus replacement implant.
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following a meniscus surgery. Prior to January 2025, orthopedic devices were not part of the TAP program, and OrthoPreserve’s device is believed to be the first orthopedic device to win TAP enrollment.
OrthoPreserve seeks to launch their pilot clinical trial in 2026, while aiming for potential FDA clearance by 2029.
The implant potentially offers an alternative to partial meniscectomy.
Brendan Baggot, Vice President of Regulatory Affairs for OrthoPreserve, said, “TAP enrollment affords enhanced guidance by agency-selected advisors on a broad range of subjects that device startups struggle with, including market adoption and insurance coverage.”
“This Breakthrough Device designation from FDA is a major milestone for OrthoPreserve and validates the longstanding unmet medical need that our meniscus implant is designed to address,” said Jonathan Schwartz, inventor, co-founder and CEO. “The anatomical design of the implant restores the normal stabilization and cushioning functions of the meniscus to relieve symptoms and preserve knee joint health.”
Source: OrthoPreserve, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







