
 Copy to clipboard
Copy to clipboard 
OrthoPediatrics (KIDS) acquired ApiFix and its minimally invasive deformity correction system, MID-C, for non-fusion treatment of progressive adolescent idiopathic scoliosis for 934,768 shares of KIDS’ common stock and $2 million in cash paid at closing, plus milestone payments and an earnout over four years.
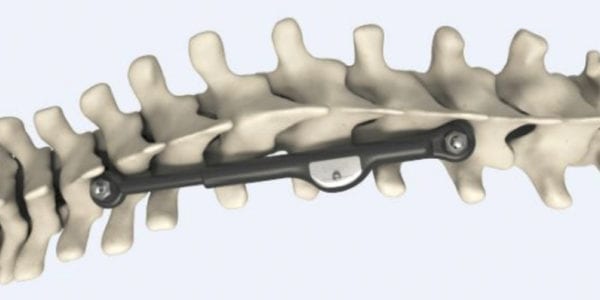
ApiFix has facilities in Israel and Boston, Massachusetts. Used with vertebral body tethering, its scoliosis system is one of two non-fusion technologies with FDA approval under its Humanitarian Device Exemption provision, joining Zimmer Biomet. MID-C is approved for use in adolescent patients with Lenke type 1 and Lenke type 5 curves of 40° to 60°, and acts as an internal brace implanted unilaterally on the concave aspect of the curvature.
The ApiFix system avoids permanently limiting range of motion and is removable. Compared to traditional fusion and tethering, MID-C offers a smaller incision (10 cm vs. standard 45 cm) and reductions in length of procedure (1-2 hours vs. 4-6), blood loss and hospitalization (1-2 days vs. 4-5 days), recovery time, complications and revision rates.
Paul Mraz, Chief Executive Officer of ApiFix, said, “The recent FDA approval of the MID-C system provides notable treatment advancements for young patients who would benefit from an alternative solution that fills the gap between non-operative therapies and irreversible spinal fusion. ApiFix’s MID-C technology is a posterior dynamic deformity correction system that enables surgeons to perform a unique treatment providing permanent curve correction while retaining spine flexibility, all via a less invasive surgical procedure. We look forward to OrthoPediatrics’ ability to increase awareness and utilization of a system that is poised to disrupt the continuum of care for scoliosis treatment in pediatric patients.”
ApiFix’s technology is protected by 46 U.S. and ex-U.S patents granted and 25 patent applications. It has an eight-year clinical history of more than 370 patients outside the U.S.
OrthoPediatrics (KIDS) acquired ApiFix and its minimally invasive deformity correction system, MID-C, for non-fusion treatment of progressive adolescent idiopathic scoliosis for 934,768 shares of KIDS' common stock and $2 million in cash paid at closing, plus milestone payments and an earnout over four years.
ApiFix has facilities in Israel...
OrthoPediatrics (KIDS) acquired ApiFix and its minimally invasive deformity correction system, MID-C, for non-fusion treatment of progressive adolescent idiopathic scoliosis for 934,768 shares of KIDS’ common stock and $2 million in cash paid at closing, plus milestone payments and an earnout over four years.
ApiFix has facilities in Israel and Boston, Massachusetts. Used with vertebral body tethering, its scoliosis system is one of two non-fusion technologies with FDA approval under its Humanitarian Device Exemption provision, joining Zimmer Biomet. MID-C is approved for use in adolescent patients with Lenke type 1 and Lenke type 5 curves of 40° to 60°, and acts as an internal brace implanted unilaterally on the concave aspect of the curvature.
The ApiFix system avoids permanently limiting range of motion and is removable. Compared to traditional fusion and tethering, MID-C offers a smaller incision (10 cm vs. standard 45 cm) and reductions in length of procedure (1-2 hours vs. 4-6), blood loss and hospitalization (1-2 days vs. 4-5 days), recovery time, complications and revision rates.
Paul Mraz, Chief Executive Officer of ApiFix, said, “The recent FDA approval of the MID-C system provides notable treatment advancements for young patients who would benefit from an alternative solution that fills the gap between non-operative therapies and irreversible spinal fusion. ApiFix’s MID-C technology is a posterior dynamic deformity correction system that enables surgeons to perform a unique treatment providing permanent curve correction while retaining spine flexibility, all via a less invasive surgical procedure. We look forward to OrthoPediatrics’ ability to increase awareness and utilization of a system that is poised to disrupt the continuum of care for scoliosis treatment in pediatric patients.”
ApiFix’s technology is protected by 46 U.S. and ex-U.S patents granted and 25 patent applications. It has an eight-year clinical history of more than 370 patients outside the U.S.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







