Copy to clipboard
Copy to clipboard 
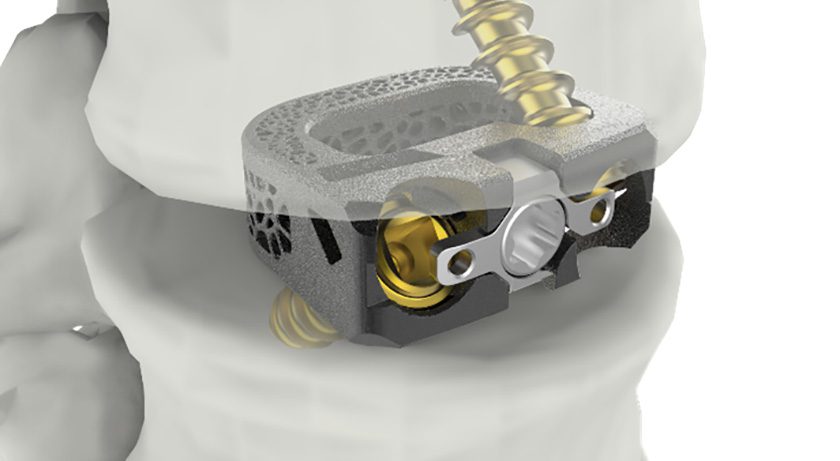
OrthoPediatrics launched BandLoc 5.5/6.0mm Sublaminar Banding technology, a complement to its RESPONSE Spine system to treat complex pathologies.
BandLoc is CE Mark approved and FDA-cleared in the U.S. and certain international markets. The pedicle-sparing band passage technique is intended to treat a variety of complex spinal pathologies, including scoliosis.
The implant incorporates the RESPONSE set screw technology and accepts multiple rod diameters. Further, BandLoc is compatible with any 5.5 or 6.0mm spinal correction system on the market and can be used with competitive systems.
Source: OrthoPediatrics Corp.; ORTHOWORLD Inc.
OrthoPediatrics launched BandLoc 5.5/6.0mm Sublaminar Banding technology, a complement to its RESPONSE Spine system to treat complex pathologies.
BandLoc is CE Mark approved and FDA-cleared in the U.S. and certain international markets. The pedicle-sparing band passage technique is intended to treat a variety of complex spinal pathologies,...
OrthoPediatrics launched BandLoc 5.5/6.0mm Sublaminar Banding technology, a complement to its RESPONSE Spine system to treat complex pathologies.
BandLoc is CE Mark approved and FDA-cleared in the U.S. and certain international markets. The pedicle-sparing band passage technique is intended to treat a variety of complex spinal pathologies, including scoliosis.
The implant incorporates the RESPONSE set screw technology and accepts multiple rod diameters. Further, BandLoc is compatible with any 5.5 or 6.0mm spinal correction system on the market and can be used with competitive systems.
Source: OrthoPediatrics Corp.; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.