Copy to clipboard
Copy to clipboard 
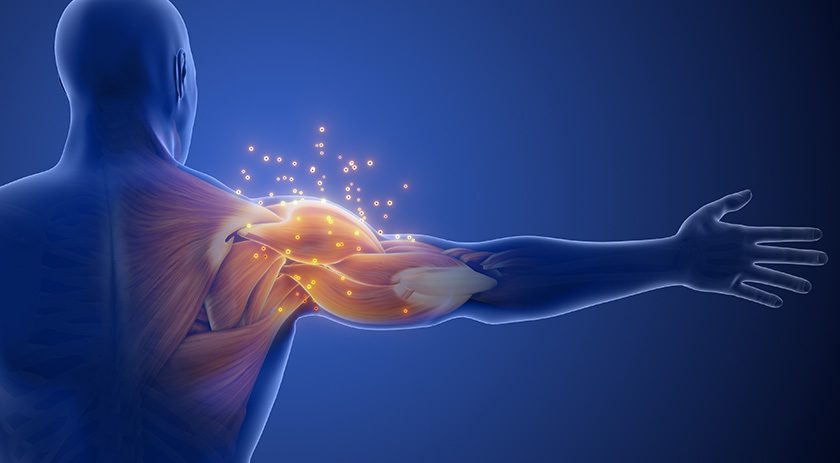
Vericel submitted a Biologics License Application to FDA for MACI™ matrix applied characterized autologous cultured chondrocytes, an investigational product intended to treat adult symptomatic cartilage defects of the knee.
Source: Vericel Corporation
The submission comes just slightly after Vericel’s previous estimate to submit the MACI BLA by the end of 2015. The product was studied in a pivotal Phase III clinical trial called SUMMIT (Superiority of MACI Implant to Microfracture Treatment) and a 3-year extension trial, results of which indicated that the treatment was clinically and statistically significantly better than microfracture, with similar structural repair tissue and safety.
Vericel submitted a Biologics License Application to FDA for MACI™ matrix applied characterized autologous cultured chondrocytes, an investigational product intended to treat adult symptomatic cartilage defects of the knee.
Source: Vericel Corporation
The submission comes just slightly after Vericel's previous estimate to submit the...
Vericel submitted a Biologics License Application to FDA for MACI™ matrix applied characterized autologous cultured chondrocytes, an investigational product intended to treat adult symptomatic cartilage defects of the knee.
Source: Vericel Corporation
The submission comes just slightly after Vericel’s previous estimate to submit the MACI BLA by the end of 2015. The product was studied in a pivotal Phase III clinical trial called SUMMIT (Superiority of MACI Implant to Microfracture Treatment) and a 3-year extension trial, results of which indicated that the treatment was clinically and statistically significantly better than microfracture, with similar structural repair tissue and safety.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.