Copy to clipboard
Copy to clipboard 
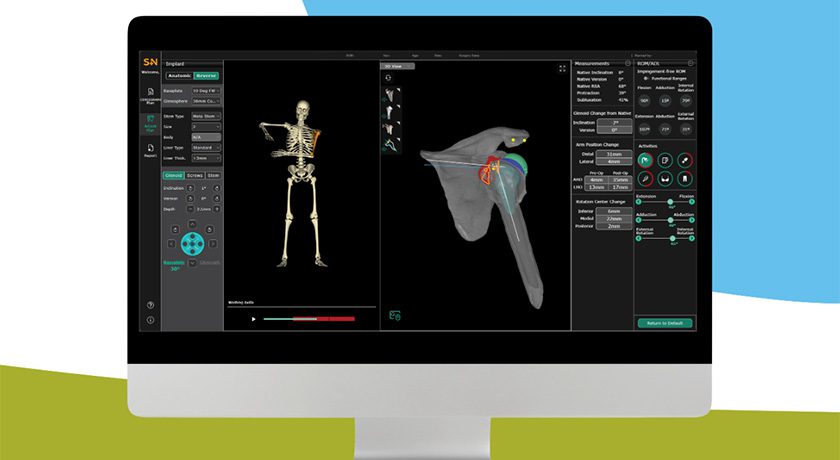
Tyber Medical received FDA 510(k) clearance of BioTy™ Modified Surface Treatment for use on its Headless Screw System. Further, the company expanded its exclusive licensing agreement with Northeastern University to further enhance BioTy proprietary technology and IP.
BioTy nano-scale technology is designed to modify the surface characteristics of medical devices for advanced indications that are yet to be disclosed, but are under development through FDA’s review process.
Source: Tyber Medical, LLC
Tyber Medical received FDA 510(k) clearance of BioTy™ Modified Surface Treatment for use on its Headless Screw System. Further, the company expanded its exclusive licensing agreement with Northeastern University to further enhance BioTy proprietary technology and IP.
BioTy nano-scale technology is designed to modify the surface...
Tyber Medical received FDA 510(k) clearance of BioTy™ Modified Surface Treatment for use on its Headless Screw System. Further, the company expanded its exclusive licensing agreement with Northeastern University to further enhance BioTy proprietary technology and IP.
BioTy nano-scale technology is designed to modify the surface characteristics of medical devices for advanced indications that are yet to be disclosed, but are under development through FDA’s review process.
Source: Tyber Medical, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.