ORTHOFLASH®
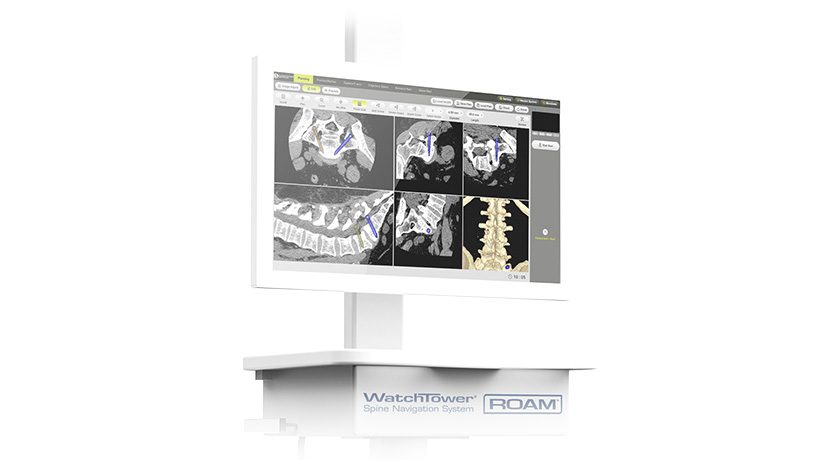
The advancement includes clearance for new 21 and 31 cm Flat-Panel C-Arm Image Calibrators and AI-assisted 2D-to-3D image registration.
The Testa TP Pivoting Spacer System is a pivoting interbody device for transforaminal lumbar interbody fusion.
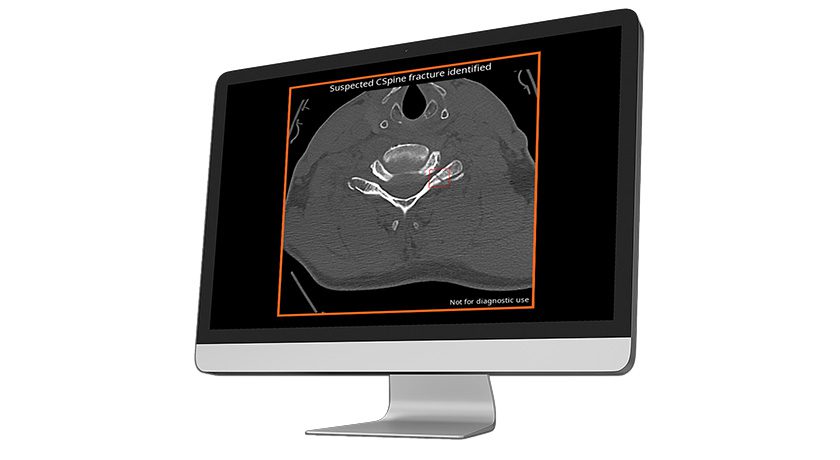
AVI is designed to host any imaging AI application, and will initially provide access to Avicenna’s entire CINA portfolio, including solutions for vertical compression and cervical spine fractures.
The advancement includes clearance for new 21 and 31 cm Flat-Panel C-Arm Image Calibrators and AI-assisted 2D-to-3D image registration.
November 13, 2025
,
The Testa TP Pivoting Spacer System is a pivoting interbody device for transforaminal lumbar interbody fusion.
November 13, 2025
,
AVI is designed to host any imaging AI application, and will initially provide access to Avicenna’s entire CINA portfolio, including solutions for vertical compression and cervical spine fractures.
November 13, 2025
,
The Hydroxyapatite Porous Polyetheretherketone biomaterial platform acts like a biologic, due to having cancellous microporosity with hydroxyapatite embedded and exposed on all surfaces.
November 13, 2025
,
The CMORE CT System comprises an enhanced set of instruments and implants for posterior stabilization of the upper spine.
November 13, 2025
,
The platform remains the only total disc replacement system offering multiple anatomically tailored implant solutions for both the cervical and lumbar spine.
November 13, 2025
,
The new clearance expands use to laminectomy and laminotomy for spinal decompression to drill through the vertebral lamina and protect patients from damage to the spinal cord.
November 13, 2025
,
The screw is indicated for fixation and stabilization of arthrodesis, osteotomies, fractures, and nonunion of the foot and ankle.
November 12, 2025
,
The next-generation headset is designed to maximize comfort, balance, and wearability in the operating room.
November 12, 2025
,
The successful procedures demonstrated the precision and efficiency of the Dynamis platform in a live clinical setting.
November 12, 2025
,
The study confirms the safety, usability, and intraoperative performance of the device across 30 patients treated in Germany.
November 12, 2025
,
The system offers a differentiated approach, combining a strong, flexible nitinol implant with a deployable prong locking mechanism.
November 12, 2025
,
The implant design reinforces the damaged labral region with a smooth, cartilage-friendly articulating surface engineered from the company’s BioPoly material.
November 7, 2025
,
The PHANTOM series joins Aevumed’s portfolio of fixation solutions designed to improve patient outcomes while supporting surgeon efficiency in the operating room.
November 7, 2025
,
The UniSpace lumbar device was designed to be compatible with CGBio’s next-generation NOVOSIS Putty bone graft substitute.