
 Copy to clipboard
Copy to clipboard 
Orthofix Medical (OFIX) received FDA Premarket Approval of the M6-C™ artificial cervical disc. Limited U.S. market launch will occur within 2019.
M6-C was developed by Spinal Kinetics, which OFIX acquired in 2018. The device received CE Mark approval in 2006 and has been implanted more than 45,000 times since then, outside of the U.S.
The approval rounds out the OFIX portfolio of cervical spine products comprising anterior, posterior and interbody fusion cervical implants, CervicalStim™ bone growth stimulation therapy and Trinity ELITE® allograft. As noted in their preliminary 2018 revenue reporting, leadership expects this launch will be a catalyst that drives the company to mid-single-digit growth in 2019 and double-digit growth in 2020.
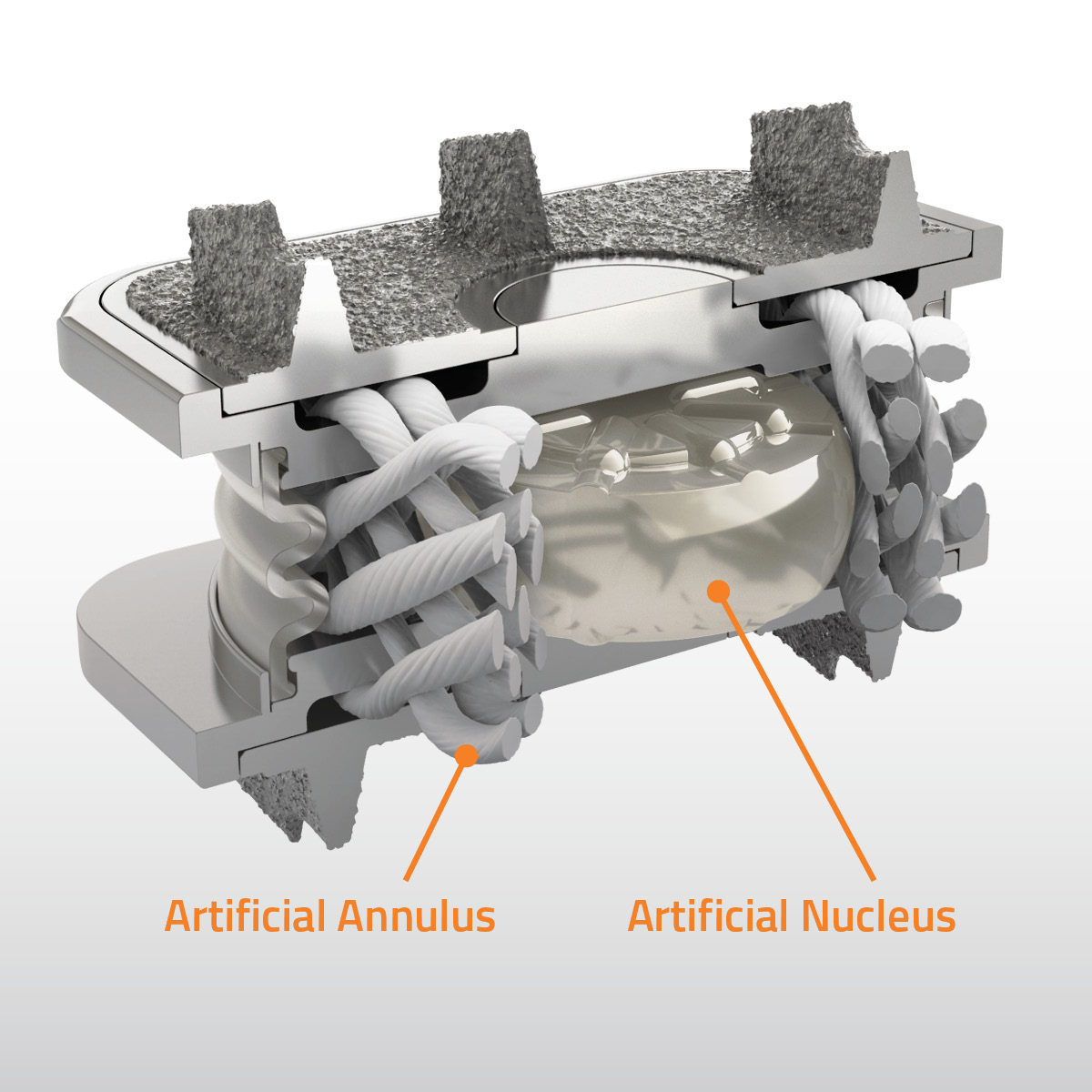
M6-C is reportedly the only artificial cervical disc designed to mimic the anatomic structure of a natural disc through use of an artificial viscoelastic nucleus and fiber annulus.
The PMA was based on clinical data from a U.S. Investigational Device Exemption study comparing M6-C to anterior cervical discectomy and fusion (ACDF) to treat cervical radiculopathy. The prospective, non-randomized, concurrently controlled, multi-center clinical trial was conducted at 23 sites.
At 24 months, results indicated:
- 90.5% of M6-C patients demonstrated a meaningful clinical improvement in the Neck Disability Index
- 91.2% clinical improvement in arm pain score for M6-C patients vs. 77.9% for ACDF
- Preservation of range of motion in flexion-extension and lateral bending in the M6-C group
- A seven times higher rate of opioid use with the ACDF patients than with patients who received M6-C, in patients still taking pain medication
- A lower rate of additional surgery needed at the treated level for M6-C vs. ACDF (1.9% vs. 4.8%)
Sources: Orthofix Medical; ORTHOWORLD Inc.
Orthofix Medical (OFIX) received FDA Premarket Approval of the M6-C™ artificial cervical disc. Limited U.S. market launch will occur within 2019.
M6-C was developed by Spinal Kinetics, which OFIX acquired in 2018. The device received CE Mark approval in 2006 and has been implanted more than 45,000 times since then, outside of the U.S.
...
Orthofix Medical (OFIX) received FDA Premarket Approval of the M6-C™ artificial cervical disc. Limited U.S. market launch will occur within 2019.
M6-C was developed by Spinal Kinetics, which OFIX acquired in 2018. The device received CE Mark approval in 2006 and has been implanted more than 45,000 times since then, outside of the U.S.
The approval rounds out the OFIX portfolio of cervical spine products comprising anterior, posterior and interbody fusion cervical implants, CervicalStim™ bone growth stimulation therapy and Trinity ELITE® allograft. As noted in their preliminary 2018 revenue reporting, leadership expects this launch will be a catalyst that drives the company to mid-single-digit growth in 2019 and double-digit growth in 2020.
M6-C is reportedly the only artificial cervical disc designed to mimic the anatomic structure of a natural disc through use of an artificial viscoelastic nucleus and fiber annulus.
The PMA was based on clinical data from a U.S. Investigational Device Exemption study comparing M6-C to anterior cervical discectomy and fusion (ACDF) to treat cervical radiculopathy. The prospective, non-randomized, concurrently controlled, multi-center clinical trial was conducted at 23 sites.
At 24 months, results indicated:
- 90.5% of M6-C patients demonstrated a meaningful clinical improvement in the Neck Disability Index
- 91.2% clinical improvement in arm pain score for M6-C patients vs. 77.9% for ACDF
- Preservation of range of motion in flexion-extension and lateral bending in the M6-C group
- A seven times higher rate of opioid use with the ACDF patients than with patients who received M6-C, in patients still taking pain medication
- A lower rate of additional surgery needed at the treated level for M6-C vs. ACDF (1.9% vs. 4.8%)
Sources: Orthofix Medical; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







