
 Copy to clipboard
Copy to clipboard 
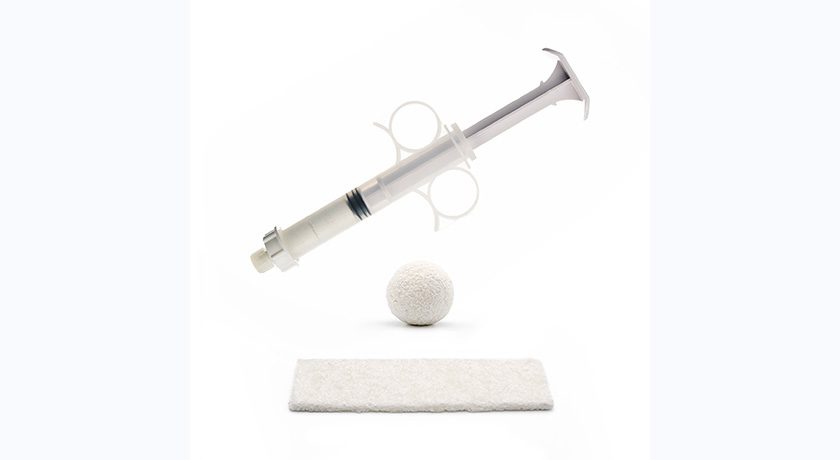
Orthofix Medical announced the FDA 510(k) clearance and full commercial launch of OsteoCove, an advanced bioactive synthetic graft. Available in both a putty and strip configuration, OsteoCove was formulated to provide superior bone-forming capabilities for a wide range of spine and orthopedic procedural applications.
Made of biphasic ceramic granules comprising beta-tricalcium phosphate and hydroxyapatite combined with type-I bovine collagen, OsteoCove features a specialized granule surface topography designed to elicit a bone-forming response, as evidenced by its ability to grow bone in a challenging muscle pouch model. This specialized surface chemistry and microporosity are shown to promote superior bone formation when compared to other commercially available advanced synthetic grafts.
Tyler Lipschultz, President, Orthofix Global Biologics, said, “With the introduction of OsteoCove, we expect strong growth in this large market segment as we continue to deliver on our commitment to provide surgeons a comprehensive offering of biologic solutions to meet the needs of their patients.”
Source: Orthofix Medical
Orthofix Medical announced the FDA 510(k) clearance and full commercial launch of OsteoCove, an advanced bioactive synthetic graft. Available in both a putty and strip configuration, OsteoCove was formulated to provide superior bone-forming capabilities for a wide range of spine and orthopedic procedural applications.
Made of biphasic ceramic...
Orthofix Medical announced the FDA 510(k) clearance and full commercial launch of OsteoCove, an advanced bioactive synthetic graft. Available in both a putty and strip configuration, OsteoCove was formulated to provide superior bone-forming capabilities for a wide range of spine and orthopedic procedural applications.
Made of biphasic ceramic granules comprising beta-tricalcium phosphate and hydroxyapatite combined with type-I bovine collagen, OsteoCove features a specialized granule surface topography designed to elicit a bone-forming response, as evidenced by its ability to grow bone in a challenging muscle pouch model. This specialized surface chemistry and microporosity are shown to promote superior bone formation when compared to other commercially available advanced synthetic grafts.
Tyler Lipschultz, President, Orthofix Global Biologics, said, “With the introduction of OsteoCove, we expect strong growth in this large market segment as we continue to deliver on our commitment to provide surgeons a comprehensive offering of biologic solutions to meet the needs of their patients.”
Source: Orthofix Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







