
 Copy to clipboard
Copy to clipboard 
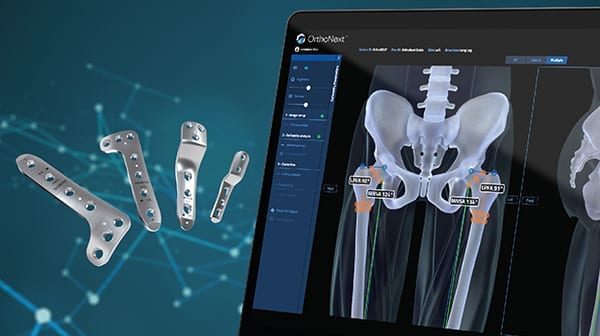
Orthofix Medical expanded its pediatric offerings with FDA 510(k) clearance of the OrthoNext™ digital platform for deformity analysis and preoperative planning of pediatric orthopedic procedures.
Developed specifically for use with JuniOrtho Plating™, OrthoNext allows surgeons to accurately plan the osteotomy position to visualize the implant in relation to anatomy. This streamlines selection of the precise size of device and enables optimal positioning for the patient’s body prior to the procedure.
Orthofix has now launched JuniOrtho Plating in the U.S. and Europe. JuniOrtho comprises sterile implants and single-use tools to optimize efficiency.
Orthofix Medical expanded its pediatric offerings with FDA 510(k) clearance of the OrthoNext™ digital platform for deformity analysis and preoperative planning of pediatric orthopedic procedures.
Developed specifically for use with JuniOrtho Plating™, OrthoNext allows surgeons to accurately plan the osteotomy position to visualize the...
Orthofix Medical expanded its pediatric offerings with FDA 510(k) clearance of the OrthoNext™ digital platform for deformity analysis and preoperative planning of pediatric orthopedic procedures.
Developed specifically for use with JuniOrtho Plating™, OrthoNext allows surgeons to accurately plan the osteotomy position to visualize the implant in relation to anatomy. This streamlines selection of the precise size of device and enables optimal positioning for the patient’s body prior to the procedure.
Orthofix has now launched JuniOrtho Plating in the U.S. and Europe. JuniOrtho comprises sterile implants and single-use tools to optimize efficiency.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







