
 Copy to clipboard
Copy to clipboard 
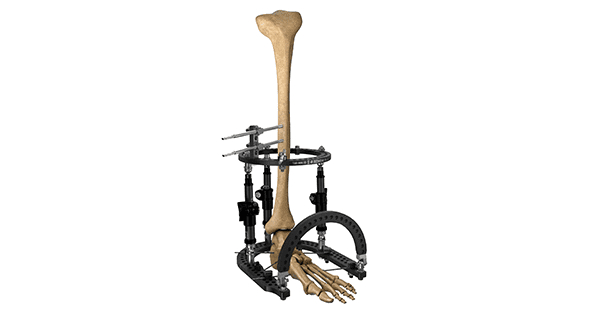
Orthofix Medical announced FDA 510(k) clearance and the first patient cases with the TrueLok™ EVO Ring Fixation System. Designed for complex limb reconstruction and deformity correction, the TrueLok EVO system is reportedly the only circular fixator on the market that features both radiolucent rings and struts to enable clear radiographic visualization.
This design allows physicians to better assess bone anatomy both during surgery and post-operative care. TrueLok EVO is also the reported to be the first circular external fixation kit available as a preassembled frame in ready-to-use single-use sterile packaging, allowing for ease of application and potential time-saving during surgery, particularly when treating post-traumatic injuries.
TrueLok EVO is made of carbon fiber, making the device lightweight for patient comfort while maintaining the same stable and versatile benefits of the original TrueLok Ring Fixation system. The system has MR conditional clearance, enabling patients, in specific conditions, to have an MRI with the fixator applied if needed.
The TrueLok EVO system is intended for fixation of open and closed fractures, limb lengthening by metaphyseal or epiphyseal distractions, treatment of nonunion or pseudarthrosis of long bones and correction of bony or soft tissue defects or deformities in adult and pediatric groups (excluding newborns). TrueLok EVO may be used in a hybrid frame construction with the ProCallus™ Fixator, XCaliber™ Fixators and the Galaxy Fixation™ system. It is also compatible with the TrueLok and TL-HEX™ systems.
Source: Orthofix Medical
Orthofix Medical announced FDA 510(k) clearance and the first patient cases with the TrueLok™ EVO Ring Fixation System. Designed for complex limb reconstruction and deformity correction, the TrueLok EVO system is reportedly the only circular fixator on the market that features both radiolucent rings and struts to enable clear radiographic...
Orthofix Medical announced FDA 510(k) clearance and the first patient cases with the TrueLok™ EVO Ring Fixation System. Designed for complex limb reconstruction and deformity correction, the TrueLok EVO system is reportedly the only circular fixator on the market that features both radiolucent rings and struts to enable clear radiographic visualization.
This design allows physicians to better assess bone anatomy both during surgery and post-operative care. TrueLok EVO is also the reported to be the first circular external fixation kit available as a preassembled frame in ready-to-use single-use sterile packaging, allowing for ease of application and potential time-saving during surgery, particularly when treating post-traumatic injuries.
TrueLok EVO is made of carbon fiber, making the device lightweight for patient comfort while maintaining the same stable and versatile benefits of the original TrueLok Ring Fixation system. The system has MR conditional clearance, enabling patients, in specific conditions, to have an MRI with the fixator applied if needed.
The TrueLok EVO system is intended for fixation of open and closed fractures, limb lengthening by metaphyseal or epiphyseal distractions, treatment of nonunion or pseudarthrosis of long bones and correction of bony or soft tissue defects or deformities in adult and pediatric groups (excluding newborns). TrueLok EVO may be used in a hybrid frame construction with the ProCallus™ Fixator, XCaliber™ Fixators and the Galaxy Fixation™ system. It is also compatible with the TrueLok and TL-HEX™ systems.
Source: Orthofix Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







