
 Copy to clipboard
Copy to clipboard 
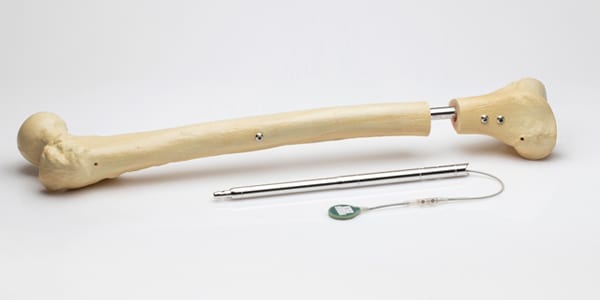
Orthofix Medical completed the acquisition of Wittenstein’s assets associated with the FITBONE® intramedullary lengthening system for limb lengthening of the femur and tibia bones. The transaction also includes potential applications in development, including the FITSPINE® system for fusionless surgery to treat early onset scoliosis.
The system includes external telemetry controls to manage distraction. Treatment is supported through an app that guides the patient throughout the process. FITBONE is FDA 510(k)-cleared and CE Mark Approved, and over 3,500 cases have been performed in more than 15 countries.
Orthofix is now reportedly the only orthopedic company offering a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction. FITBONE joins the Orthofix Extremities portfolio that includes the TL-HEX™ computer-assisted ring fixation system for external limb lengthening and the eight-Plate Guided Growth System™ for correcting angular growth deformities in pediatric patients.
“The acquisition of the FITBONE® intramedullary lengthening system further demonstrates Orthofix’s commitment to investing in differentiated products that are a strong strategic fit within our core businesses,” said Orthofix President and Chief Executive Officer Jon Serbousek. “Adding this technology to our current limb reconstruction portfolio enables us to offer physicians solutions to meet the needs of their patients which may require internal or external fixation procedures. We look forward to developing other applications of this exciting platform.”
Orthofix Medical completed the acquisition of Wittenstein's assets associated with the FITBONE® intramedullary lengthening system for limb lengthening of the femur and tibia bones. The transaction also includes potential applications in development, including the FITSPINE® system for fusionless surgery to treat early onset...
Orthofix Medical completed the acquisition of Wittenstein’s assets associated with the FITBONE® intramedullary lengthening system for limb lengthening of the femur and tibia bones. The transaction also includes potential applications in development, including the FITSPINE® system for fusionless surgery to treat early onset scoliosis.
The system includes external telemetry controls to manage distraction. Treatment is supported through an app that guides the patient throughout the process. FITBONE is FDA 510(k)-cleared and CE Mark Approved, and over 3,500 cases have been performed in more than 15 countries.
Orthofix is now reportedly the only orthopedic company offering a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction. FITBONE joins the Orthofix Extremities portfolio that includes the TL-HEX™ computer-assisted ring fixation system for external limb lengthening and the eight-Plate Guided Growth System™ for correcting angular growth deformities in pediatric patients.
“The acquisition of the FITBONE® intramedullary lengthening system further demonstrates Orthofix’s commitment to investing in differentiated products that are a strong strategic fit within our core businesses,” said Orthofix President and Chief Executive Officer Jon Serbousek. “Adding this technology to our current limb reconstruction portfolio enables us to offer physicians solutions to meet the needs of their patients which may require internal or external fixation procedures. We look forward to developing other applications of this exciting platform.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
ME
Mike Evers is a Senior Market Analyst and writer with over 15 years of experience in the medical industry, spanning cardiac rhythm management, ER coding and billing, and orthopedics. He joined ORTHOWORLD in 2018, where he provides market analysis and editorial coverage.







