Copy to clipboard
Copy to clipboard 
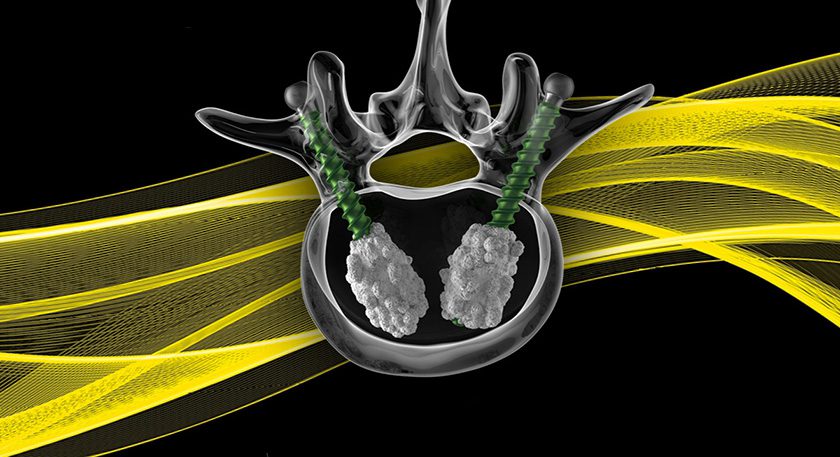
Orthocell was granted ethical approval by St Vincent’s Hospital Melbourne, Australia, for a clinical study using the Celgro SMRT Graft collagen scaffold.
Launching in 2Q16, the 25-patient study will investigate safety and tolerability of Celgro as a collagen matrix treatment for osteochondral defects in the hip—specifically, an enhanced version of the microfracture technique.
Celgro technology has been granted patent protection in China and the U.S., and since 4Q14, Orthocell and BONESUPPORT are co-developing bone repair products based on Celgro and CERAMENT™ injectable synthetic bone substitute to create a novel product that will not only support damaged bone, but induce the growth of new bone matrix.
Sources: Life Scientist, ORTHOWORLD Inc.
Orthocell was granted ethical approval by St Vincent’s Hospital Melbourne, Australia, for a clinical study using the Celgro SMRT Graft collagen scaffold.
Launching in 2Q16, the 25-patient study will investigate safety and tolerability of Celgro as a collagen matrix treatment for osteochondral defects in the hip—specifically, an enhanced...
Orthocell was granted ethical approval by St Vincent’s Hospital Melbourne, Australia, for a clinical study using the Celgro SMRT Graft collagen scaffold.
Launching in 2Q16, the 25-patient study will investigate safety and tolerability of Celgro as a collagen matrix treatment for osteochondral defects in the hip—specifically, an enhanced version of the microfracture technique.
Celgro technology has been granted patent protection in China and the U.S., and since 4Q14, Orthocell and BONESUPPORT are co-developing bone repair products based on Celgro and CERAMENT™ injectable synthetic bone substitute to create a novel product that will not only support damaged bone, but induce the growth of new bone matrix.
Sources: Life Scientist, ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.