
 Copy to clipboard
Copy to clipboard 
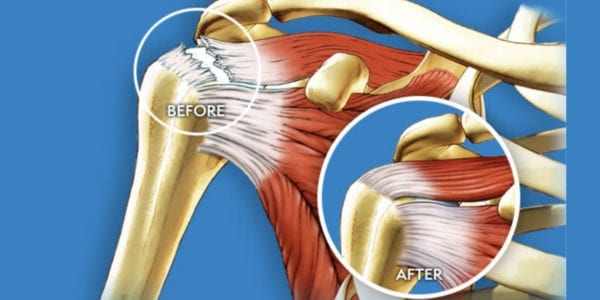
Ortho Regenerative Technologies (Ortho RTI) announced positive results following completion of a pivotal preclinical study in rotator cuff tear repair. Results confirmed the safety profile of ORTHO-R biopolymer as well as statistical significance over standard-of-care (sutures and anchors) alone.
Ortho RTI has completed two studies in large animals, namely, a pilot study with 18 sheep with MRI data collected at 6 weeks and 3 months and histopathology at 3 months, then, a pivotal study with 48 sheep collecting MRI data at 3 and 6 months and histopathology at 6 months. Treatment with anchors and sutures was compared to standard of care plus ORTHO-R muco-adhesive orthobiologic hybrid implant.
Pilot and pivotal studies demonstrated a decrease in tendon gap at 3 months in ORTHO-R treated groups vs. standard of care control groups. A decreased tendon gap is indicative of faster restauration of tissue structure. In both studies, results at 3 months and 6 months showed equivalent safety.
Ortho RTI’s platform acts as a biodegradable scaffold that retains any type of bioactive material, prolonging its therapeutic effect and improving benefits to patients. Ortho-R is mixed with Platelet-Rich Plasma to deliver biologics that can increase the healing rates of occupational and sports related injuries to tendons, meniscus, ligaments and cartilage. The polymer/biologics combination can be directly applied to the site of injury during a routine operative procedure without significantly extending the time of the surgery and without further intervention.
“These positive preclinical results are the crowning of almost two years of hard work from ORTHO RTI’s team and CRO collaborators, with the financial support from our investors and shareholders,” said Mr. Claude LeDuc, CEO. “We are now transitioning from preclinical to clinical stage…All our efforts are now focused on completing the ongoing regulatory process to start enrolling patients in our planned U.S. Rotator Cuff Tear repair clinical trial.”
Ortho Regenerative Technologies (Ortho RTI) announced positive results following completion of a pivotal preclinical study in rotator cuff tear repair. Results confirmed the safety profile of ORTHO-R biopolymer as well as statistical significance over standard-of-care (sutures and anchors) alone.
Ortho RTI has completed two studies in large...
Ortho Regenerative Technologies (Ortho RTI) announced positive results following completion of a pivotal preclinical study in rotator cuff tear repair. Results confirmed the safety profile of ORTHO-R biopolymer as well as statistical significance over standard-of-care (sutures and anchors) alone.
Ortho RTI has completed two studies in large animals, namely, a pilot study with 18 sheep with MRI data collected at 6 weeks and 3 months and histopathology at 3 months, then, a pivotal study with 48 sheep collecting MRI data at 3 and 6 months and histopathology at 6 months. Treatment with anchors and sutures was compared to standard of care plus ORTHO-R muco-adhesive orthobiologic hybrid implant.
Pilot and pivotal studies demonstrated a decrease in tendon gap at 3 months in ORTHO-R treated groups vs. standard of care control groups. A decreased tendon gap is indicative of faster restauration of tissue structure. In both studies, results at 3 months and 6 months showed equivalent safety.
Ortho RTI’s platform acts as a biodegradable scaffold that retains any type of bioactive material, prolonging its therapeutic effect and improving benefits to patients. Ortho-R is mixed with Platelet-Rich Plasma to deliver biologics that can increase the healing rates of occupational and sports related injuries to tendons, meniscus, ligaments and cartilage. The polymer/biologics combination can be directly applied to the site of injury during a routine operative procedure without significantly extending the time of the surgery and without further intervention.
“These positive preclinical results are the crowning of almost two years of hard work from ORTHO RTI’s team and CRO collaborators, with the financial support from our investors and shareholders,” said Mr. Claude LeDuc, CEO. “We are now transitioning from preclinical to clinical stage…All our efforts are now focused on completing the ongoing regulatory process to start enrolling patients in our planned U.S. Rotator Cuff Tear repair clinical trial.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







