
 Copy to clipboard
Copy to clipboard 
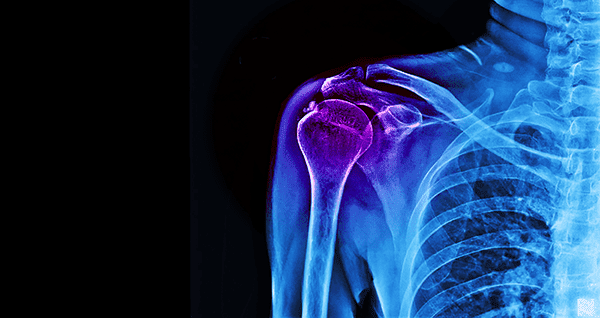
Ortho Regenerative Technologies (ORT) entered into a Material Transfer Agreement with an undisclosed medical device company for the exclusive use of their proprietary platelet-rich plasma (PRP) system in ORT’s upcoming Phase I/II U.S. clinical trial of ORTHO-R rotator cuff tear repair.
The company will provide the PRP system disposable kits for all enrolled patients in the study. In return, Ortho has provided the company with a right of first offer to distribute ORTHO-R in alongside their proprietary PRP System.
The ORTHO-R Phase I/II clinical study is a prospective, randomized, controlled and blinded clinical trial, to evaluate ORTHO-R as an adjunct to standard of care surgery vs. standard of care surgery alone for rotator cuff tear repair. The clinical trial will enroll 78 patients at ten clinical sites.
FDA placed the clinical trial on hold in June 2021. The hold was lifted in December.
The ORTHO-R/PRP combination product has demonstrated quicker and better tissue repair than the standard of care surgery in a preclinical rotator cuff repair study.
Claude LeDuc, President and CEO, said, “Our ORTHO-R U.S. clinical trial will be one of the first FDA regulated Drug/Biologics combination product clinical trials in the orthobiologics field. We are making significant progress with pre-enrollment activities at each respective site. We are confident to start enrolling our first patients during the coming months.”
Ortho Regenerative Technologies (ORT) entered into a Material Transfer Agreement with an undisclosed medical device company for the exclusive use of their proprietary platelet-rich plasma (PRP) system in ORT's upcoming Phase I/II U.S. clinical trial of ORTHO-R rotator cuff tear repair.
The company will provide the PRP system disposable kits for...
Ortho Regenerative Technologies (ORT) entered into a Material Transfer Agreement with an undisclosed medical device company for the exclusive use of their proprietary platelet-rich plasma (PRP) system in ORT’s upcoming Phase I/II U.S. clinical trial of ORTHO-R rotator cuff tear repair.
The company will provide the PRP system disposable kits for all enrolled patients in the study. In return, Ortho has provided the company with a right of first offer to distribute ORTHO-R in alongside their proprietary PRP System.
The ORTHO-R Phase I/II clinical study is a prospective, randomized, controlled and blinded clinical trial, to evaluate ORTHO-R as an adjunct to standard of care surgery vs. standard of care surgery alone for rotator cuff tear repair. The clinical trial will enroll 78 patients at ten clinical sites.
FDA placed the clinical trial on hold in June 2021. The hold was lifted in December.
The ORTHO-R/PRP combination product has demonstrated quicker and better tissue repair than the standard of care surgery in a preclinical rotator cuff repair study.
Claude LeDuc, President and CEO, said, “Our ORTHO-R U.S. clinical trial will be one of the first FDA regulated Drug/Biologics combination product clinical trials in the orthobiologics field. We are making significant progress with pre-enrollment activities at each respective site. We are confident to start enrolling our first patients during the coming months.”
Source: Ortho Regenerative Technologies Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.