
 Copy to clipboard
Copy to clipboard 
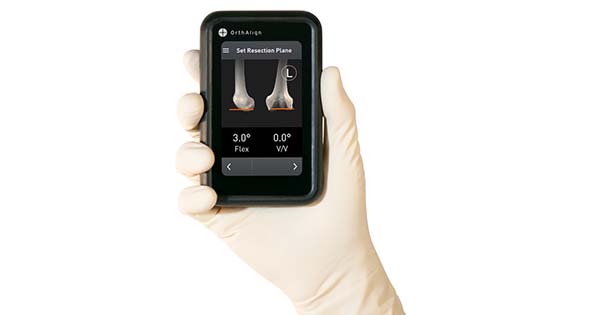
OrthAlign commenced full market launch of Lantern smart surgical technology for total and partial knee replacement surgery.
Lantern delivers individualized alignment to any patient with a single-use, handheld smart tool that can be used across implant platforms and surgical philosophies. The user-friendly design and interface provide streamlined workflows to reduce O.R. times and support multiple O.R.s concurrently without the investment, equipment or pre-operative imaging required by many computer-assisted surgical systems. The technology is optimized for the ASC with only one instrument tray, one navigation unit for all applications, and no storage requirements or service plans.
Lantern received FDA 510(k) clearance in 4Q20 and has been available in limited settings since. Lantern currently supports total knee and unicompartmental knee arthroplasty with developing applications for total hip arthroplasty, intraoperative assessment tools such as soft tissue balancing, data collection capabilities, and network connectivity.
Source: OrthAlign, Inc.
OrthAlign commenced full market launch of Lantern smart surgical technology for total and partial knee replacement surgery.
Lantern delivers individualized alignment to any patient with a single-use, handheld smart tool that can be used across implant platforms and surgical philosophies. The user-friendly design and interface provide streamlined...
OrthAlign commenced full market launch of Lantern smart surgical technology for total and partial knee replacement surgery.
Lantern delivers individualized alignment to any patient with a single-use, handheld smart tool that can be used across implant platforms and surgical philosophies. The user-friendly design and interface provide streamlined workflows to reduce O.R. times and support multiple O.R.s concurrently without the investment, equipment or pre-operative imaging required by many computer-assisted surgical systems. The technology is optimized for the ASC with only one instrument tray, one navigation unit for all applications, and no storage requirements or service plans.
Lantern received FDA 510(k) clearance in 4Q20 and has been available in limited settings since. Lantern currently supports total knee and unicompartmental knee arthroplasty with developing applications for total hip arthroplasty, intraoperative assessment tools such as soft tissue balancing, data collection capabilities, and network connectivity.
Source: OrthAlign, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







