Copy to clipboard
Copy to clipboard 
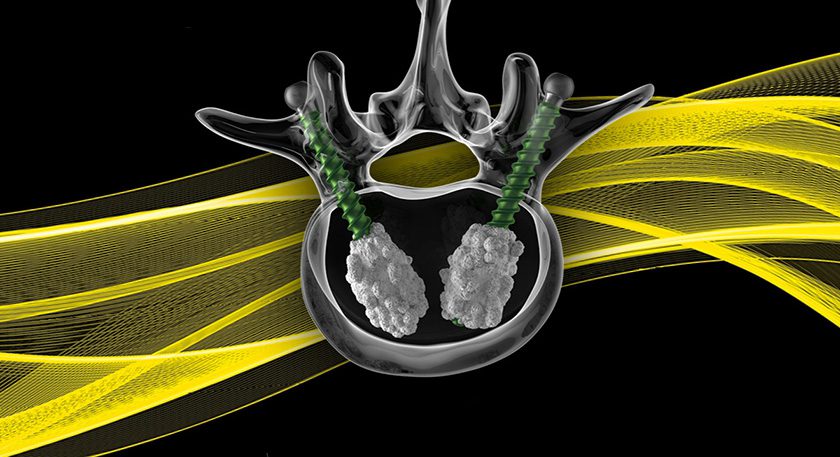
Onkos Surgical received FDA 510(k) clearance for its modular collar portfolio featuring BioGrip® 3D-printed implant technology. The patented design, now available as part of the ELEOS™ Limb Salvage System, was created to help address the clinical challenge of aseptic loosening (also known as implant loosening) in musculoskeletal oncology and complex orthopaedic limb salvage surgery.
With its new 3D-printed porous structure and novel Nano HA treatment, the BioGrip collar is designed to help provide bone ingrowth at the bone-implant interface which may help address aseptic loosening. The treatment helps to accelerate and enhance osseointegration.
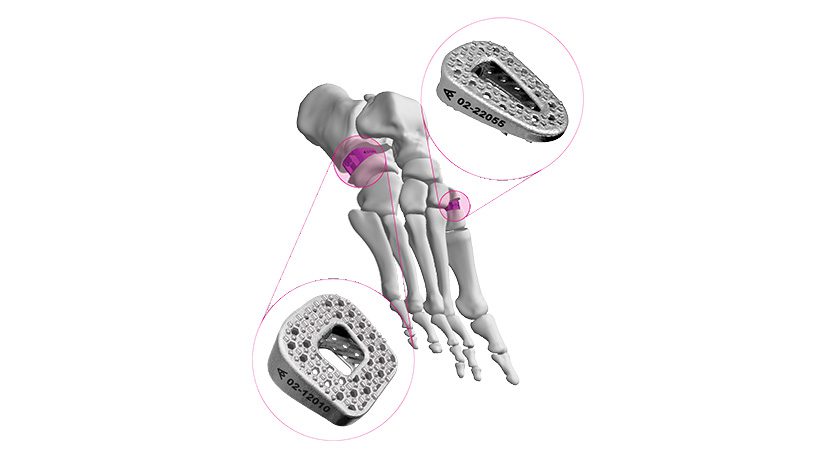
The 3D-printed, nano HA treated design is one of two interchangeable collar designs that Onkos has added to its offering to address these complex clinical challenges. The clearance also includes an oval shaped collar design to provide greater contact in distal femoral replacements, where subsidence is a common challenge among complex revision and trauma applications.
Patrick Treacy, CEO and co-founder of Onkos Surgical, said, “This is a historic day for Onkos Surgical. When we started the company, we set out to deliver innovation that would directly address the long-established clinical challenges of limb salvage surgery – soft-tissue attachment, implant loosening, and infection. In the past six months, we have made significant progress by launching new ELEOS products aimed at improving the challenges of soft tissue attachment. Our latest innovation, the BioGrip collar portfolio, delivers on our promise to help address the challenge of implant loosening. With our focused commercial approach and substantial R&D investments, we will continue to be the leading provider of innovative solutions for musculoskeletal oncology and other complex orthopaedic procedures.”
Source: Onkos Surgical
Onkos Surgical received FDA 510(k) clearance for its modular collar portfolio featuring BioGrip® 3D-printed implant technology. The patented design, now available as part of the ELEOS™ Limb Salvage System, was created to help address the clinical challenge of aseptic loosening (also known as implant loosening) in musculoskeletal oncology and...
Onkos Surgical received FDA 510(k) clearance for its modular collar portfolio featuring BioGrip® 3D-printed implant technology. The patented design, now available as part of the ELEOS™ Limb Salvage System, was created to help address the clinical challenge of aseptic loosening (also known as implant loosening) in musculoskeletal oncology and complex orthopaedic limb salvage surgery.
With its new 3D-printed porous structure and novel Nano HA treatment, the BioGrip collar is designed to help provide bone ingrowth at the bone-implant interface which may help address aseptic loosening. The treatment helps to accelerate and enhance osseointegration.
The 3D-printed, nano HA treated design is one of two interchangeable collar designs that Onkos has added to its offering to address these complex clinical challenges. The clearance also includes an oval shaped collar design to provide greater contact in distal femoral replacements, where subsidence is a common challenge among complex revision and trauma applications.
Patrick Treacy, CEO and co-founder of Onkos Surgical, said, “This is a historic day for Onkos Surgical. When we started the company, we set out to deliver innovation that would directly address the long-established clinical challenges of limb salvage surgery – soft-tissue attachment, implant loosening, and infection. In the past six months, we have made significant progress by launching new ELEOS products aimed at improving the challenges of soft tissue attachment. Our latest innovation, the BioGrip collar portfolio, delivers on our promise to help address the challenge of implant loosening. With our focused commercial approach and substantial R&D investments, we will continue to be the leading provider of innovative solutions for musculoskeletal oncology and other complex orthopaedic procedures.”
Source: Onkos Surgical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.