Copy to clipboard
Copy to clipboard 
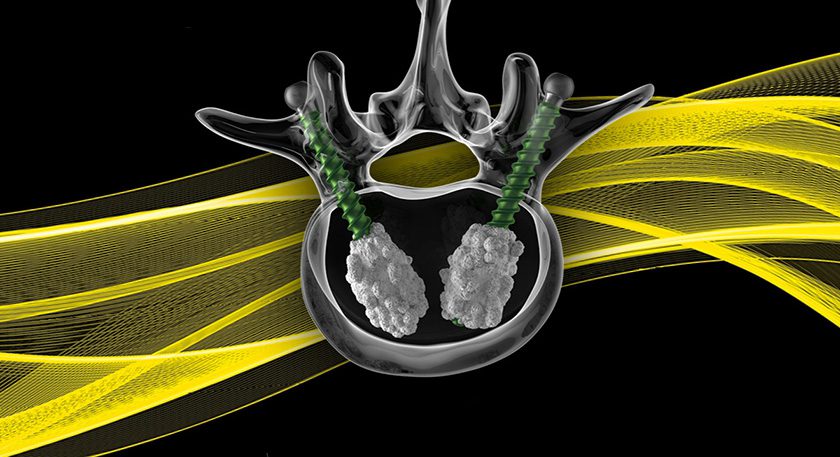
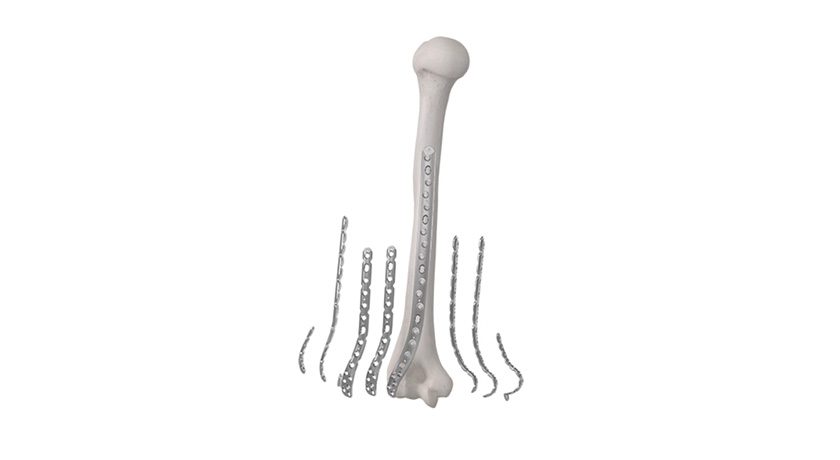
Onkos Surgical was granted FDA 510(k) clearance to market its ELEOS Proximal Tibia with NanoCept Antibacterial Technology. This marks the first 510(k) clearance for the NanoCept Antibacterial Technology since the original De Novo authorization was granted in April 2024.
NanoCept technology offers a proactive approach against intraoperative bacterial contamination. In preclinical studies supporting the original De Novo market authorization, NanoCept demonstrated up to a 99.999% kill rate of bacteria that are commonly found in the O.R.
The ELEOS Proximal Tibia with NanoCept Antibacterial Technology is part of the company’s ELEOS Limb Salvage System, which offers comprehensive reconstruction options for patients with significant bone loss due to cancer, trauma, or previous surgical procedures.
“This first post-De Novo 510(k) clearance marks a major milestone, highlighting our commitment and capability to advance NanoCept technology throughout the ELEOS implant system—and ultimately beyond,” said Patrick Treacy, CEO and Co-founder of Onkos. “We remain dedicated to supporting the surgeons we serve and the patients they treat by delivering truly innovative and differentiated technologies.”
Source: Onkos Surgical
Onkos Surgical was granted FDA 510(k) clearance to market its ELEOS Proximal Tibia with NanoCept Antibacterial Technology. This marks the first 510(k) clearance for the NanoCept Antibacterial Technology since the original De Novo authorization was granted in April 2024.
NanoCept technology offers a proactive approach against intraoperative...
Onkos Surgical was granted FDA 510(k) clearance to market its ELEOS Proximal Tibia with NanoCept Antibacterial Technology. This marks the first 510(k) clearance for the NanoCept Antibacterial Technology since the original De Novo authorization was granted in April 2024.
NanoCept technology offers a proactive approach against intraoperative bacterial contamination. In preclinical studies supporting the original De Novo market authorization, NanoCept demonstrated up to a 99.999% kill rate of bacteria that are commonly found in the O.R.
The ELEOS Proximal Tibia with NanoCept Antibacterial Technology is part of the company’s ELEOS Limb Salvage System, which offers comprehensive reconstruction options for patients with significant bone loss due to cancer, trauma, or previous surgical procedures.
“This first post-De Novo 510(k) clearance marks a major milestone, highlighting our commitment and capability to advance NanoCept technology throughout the ELEOS implant system—and ultimately beyond,” said Patrick Treacy, CEO and Co-founder of Onkos. “We remain dedicated to supporting the surgeons we serve and the patients they treat by delivering truly innovative and differentiated technologies.”
Source: Onkos Surgical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.