Copy to clipboard
Copy to clipboard 
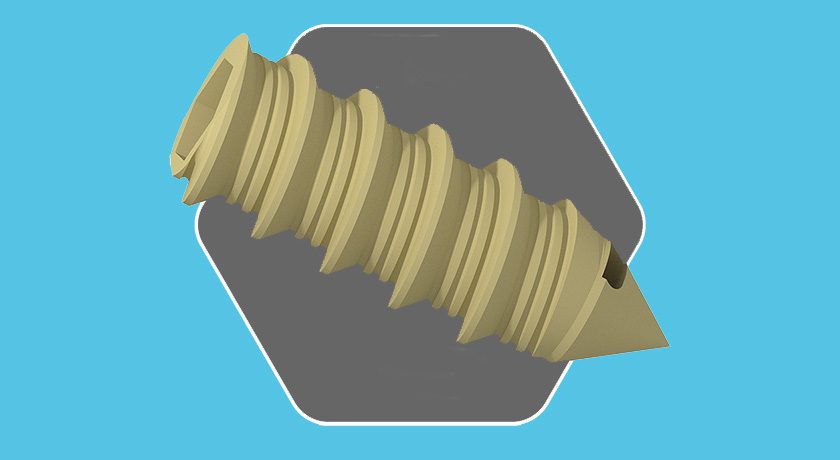
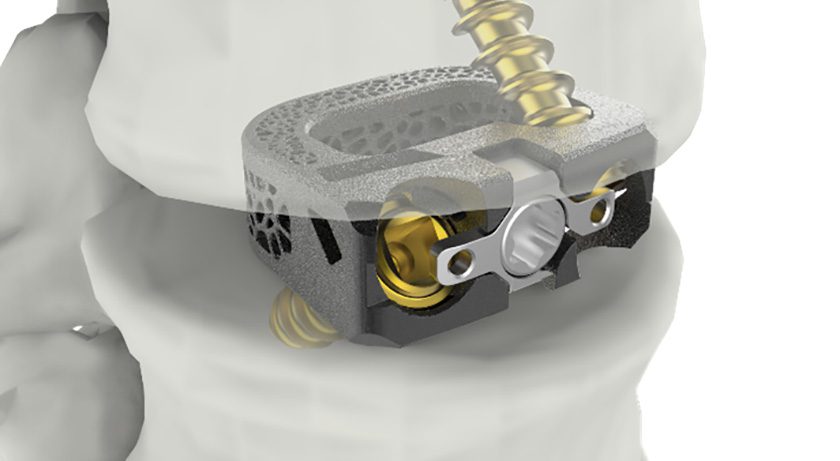
 Omnia Medical received FDA 510(k) clearance to market its vertebral body replacement in the thoracolumbar spine. This is reportedly the first PEEK-OPTIMA HA VBR to receive FDA clearance.
Omnia Medical received FDA 510(k) clearance to market its vertebral body replacement in the thoracolumbar spine. This is reportedly the first PEEK-OPTIMA HA VBR to receive FDA clearance.
The device, manufactured from PEEK-OPTIMA™ HA Enhanced polymer, is the product of collaboration among Omnia, JALEX Medical and Invibio Biomaterial Solutions. JALEX provided product development and regulatory support, while Invibio is the provider of PEEK material.
Early clinical results have demonstrated solid fusion with dense bone apposition at 6 months and improvements in overall pain and neurological function with use of a PEEK-OPTIMA HA Enhanced interbody fusion device. Other fusion devices based on HA Enhanced PEEK-OPTIMA include:
- Innovasis‘ Ax™ standalone system
- Meditech Spine’s Talos®
- Pinnacle Spine’s InFill®
- Spineology’s Rampart One
Sources: Omnia Medical; ORTHOWORLD Inc.
Omnia Medical received FDA 510(k) clearance to market its vertebral body replacement in the thoracolumbar spine. This is reportedly the first PEEK-OPTIMA HA VBR to receive FDA clearance.
The device, manufactured from PEEK-OPTIMA™ HA Enhanced polymer, is the product of collaboration among Omnia, JALEX Medical and Invibio Biomaterial Solutions....
 Omnia Medical received FDA 510(k) clearance to market its vertebral body replacement in the thoracolumbar spine. This is reportedly the first PEEK-OPTIMA HA VBR to receive FDA clearance.
Omnia Medical received FDA 510(k) clearance to market its vertebral body replacement in the thoracolumbar spine. This is reportedly the first PEEK-OPTIMA HA VBR to receive FDA clearance.
The device, manufactured from PEEK-OPTIMA™ HA Enhanced polymer, is the product of collaboration among Omnia, JALEX Medical and Invibio Biomaterial Solutions. JALEX provided product development and regulatory support, while Invibio is the provider of PEEK material.
Early clinical results have demonstrated solid fusion with dense bone apposition at 6 months and improvements in overall pain and neurological function with use of a PEEK-OPTIMA HA Enhanced interbody fusion device. Other fusion devices based on HA Enhanced PEEK-OPTIMA include:
- Innovasis‘ Ax™ standalone system
- Meditech Spine’s Talos®
- Pinnacle Spine’s InFill®
- Spineology’s Rampart One
Sources: Omnia Medical; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.