
 Copy to clipboard
Copy to clipboard 
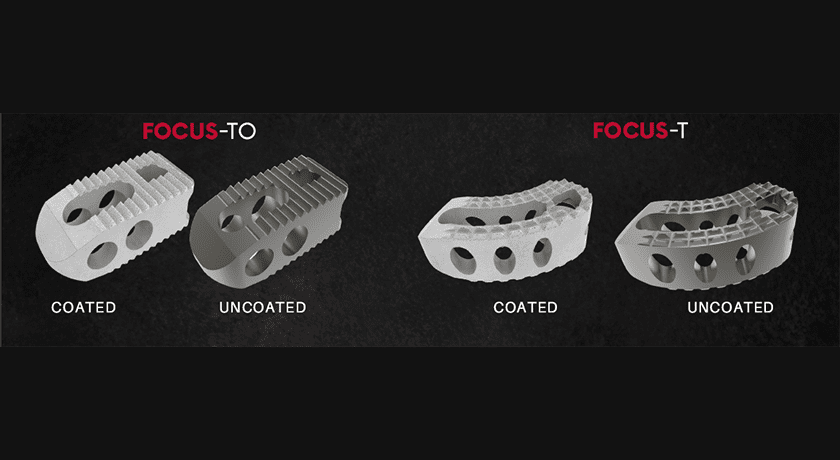
OC Spine, a division of OC Medical Devices, was granted FDA 510(k) clearance to market its Focus family of Ti TLIF interbody devices featuring xCELLerate bioactive surface technology.
Focus will be available in both oblique and banana style options and is cleared for use with or without the xCELLerate technology. The hydrophilic nature of xCELLerate has been shown to attract and absorb blood, increasing the growth factors needed to promote the integration of bone to the implant.
OC Spine is a division of OC Medical Devices.
Jason Bazemore, VP of Sales & Marketing, said, “We are excited to have clearance of the Focus line and also thrilled that this paves the way for incorporating our xCELLerate technology on future implant systems in our development pipeline.”
Source: OC Medical Devices
OC Spine, a division of OC Medical Devices, was granted FDA 510(k) clearance to market its Focus family of Ti TLIF interbody devices featuring xCELLerate bioactive surface technology.
Focus will be available in both oblique and banana style options and is cleared for use with or without the xCELLerate technology. The hydrophilic nature of...
OC Spine, a division of OC Medical Devices, was granted FDA 510(k) clearance to market its Focus family of Ti TLIF interbody devices featuring xCELLerate bioactive surface technology.
Focus will be available in both oblique and banana style options and is cleared for use with or without the xCELLerate technology. The hydrophilic nature of xCELLerate has been shown to attract and absorb blood, increasing the growth factors needed to promote the integration of bone to the implant.
OC Spine is a division of OC Medical Devices.
Jason Bazemore, VP of Sales & Marketing, said, “We are excited to have clearance of the Focus line and also thrilled that this paves the way for incorporating our xCELLerate technology on future implant systems in our development pipeline.”
Source: OC Medical Devices

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







