
 Copy to clipboard
Copy to clipboard 
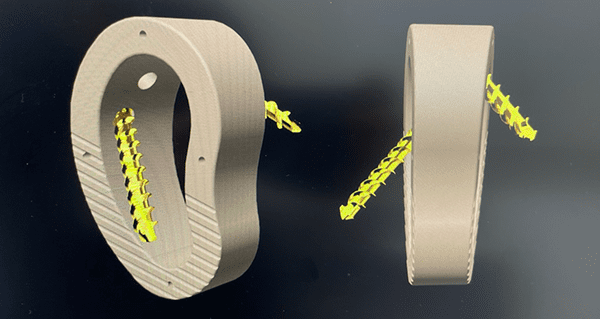
Nvision Biomedical Technologies received FDA 510(k) clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision that use PEEK-OPTIMA HA Enhanced. This polymer from Invibio Biomaterial Solutions promotes multi-directional bone healing and allows for improved fixation. Additionally, the Trigon Lapidus Wedge is the first implant to specifically reference lengthening in its FDA indication.
The Trigon Lapidus Wedge is indicated for a first metatarsal-cuneiform lengthening arthrodesis. It is offered in three footprint sizes with various length restoring thicknesses, as well as variations in sagittal and transverse angle correction. Additionally, the system provides a jig that allows for frontal plane rotation, resulting in triplanar correction with the ability to restore or maintain length of the first metatarsal.
The Trigon Stand-Alone Subtalar Wedge is the first PEEK-OPTIMA HA Enhanced wedge for Subtalar Fusion in the market. The PEEK-OPTIMA HA Enhanced material, which has a modulus similar to bone, allows artifact-free imaging and provides an osteoconductive surface for bone ongrowth. With a 25mm diameter, the Subtalar Wedge offers correction heights ranging from 6mm to 16mm in parallel and angled options.
In the last four months, Nvision added four products with FDA clearance to its portfolio, with one currently in FDA review and two more scheduled for submission. Nvision has 18 issued patents, with more than 30 in development.
Source: Nvision Biomedical Technologies
Nvision Biomedical Technologies received FDA 510(k) clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision that use PEEK-OPTIMA HA Enhanced. This polymer from Invibio Biomaterial...
Nvision Biomedical Technologies received FDA 510(k) clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision that use PEEK-OPTIMA HA Enhanced. This polymer from Invibio Biomaterial Solutions promotes multi-directional bone healing and allows for improved fixation. Additionally, the Trigon Lapidus Wedge is the first implant to specifically reference lengthening in its FDA indication.
The Trigon Lapidus Wedge is indicated for a first metatarsal-cuneiform lengthening arthrodesis. It is offered in three footprint sizes with various length restoring thicknesses, as well as variations in sagittal and transverse angle correction. Additionally, the system provides a jig that allows for frontal plane rotation, resulting in triplanar correction with the ability to restore or maintain length of the first metatarsal.
The Trigon Stand-Alone Subtalar Wedge is the first PEEK-OPTIMA HA Enhanced wedge for Subtalar Fusion in the market. The PEEK-OPTIMA HA Enhanced material, which has a modulus similar to bone, allows artifact-free imaging and provides an osteoconductive surface for bone ongrowth. With a 25mm diameter, the Subtalar Wedge offers correction heights ranging from 6mm to 16mm in parallel and angled options.
In the last four months, Nvision added four products with FDA clearance to its portfolio, with one currently in FDA review and two more scheduled for submission. Nvision has 18 issued patents, with more than 30 in development.
Source: Nvision Biomedical Technologies

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







