
 Copy to clipboard
Copy to clipboard 
Nvision Biomedical Technologies received FDA 510(k) clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision utilizing PEEK-OPTIMA HA Enhanced. This polymer from Invibio Biomaterial Solutions promotes multi-directional bone healing and allows for improved fixation. Additionally, the Trigon Lapidus Wedge is the first implant to specifically reference lengthening in its FDA indication.
The Trigon Lapidus Wedge is a PEEK-OPTIMA HA Enhanced implant indicated for a first metatarsal-cuneiform lengthening arthrodesis. It is offered in three footprint sizes with various length restoring thicknesses, as well as variations in sagittal and transverse angle correction. Additionally, the system provides a jig that allows for frontal plane rotation, resulting in triplanar correction with the ability to restore or maintain length of the first metatarsal.
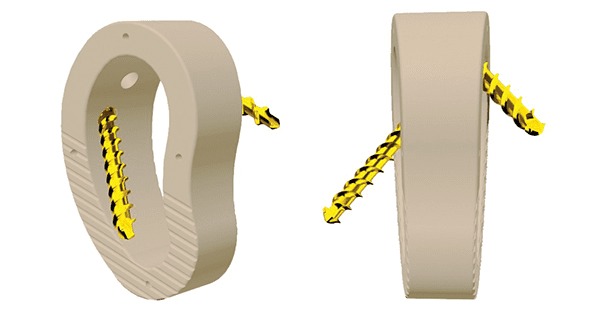
The Trigon Stand-Alone Subtalar Wedge is the first PEEK-OPTIMA HA Enhanced wedge for Subtalar Fusion in the market. The PEEK-OPTIMA HA Enhanced material, which has a modulus similar to bone, allows artifact-free imaging and provides an osteoconductive surface for bone ongrowth. With a 25mm diameter, the Subtalar Wedge offers correction heights ranging from 6mm to 16mm in parallel and angled options.
In the last four months, Nvision added four products with FDA clearance to its portfolio, with one currently in FDA review and two more scheduled for submission.
Source: Nvision Biomedical Technologies
Nvision Biomedical Technologies received FDA 510(k) clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision utilizing PEEK-OPTIMA HA Enhanced. This polymer from Invibio Biomaterial...
Nvision Biomedical Technologies received FDA 510(k) clearance for the first use of PEEK-OPTIMA® HA Enhanced in Lapidus and Subtalar Fusion Wedges. Through the expansion of its Trigon® product line, these wedges are the fifth and sixth medical devices cleared by Nvision utilizing PEEK-OPTIMA HA Enhanced. This polymer from Invibio Biomaterial Solutions promotes multi-directional bone healing and allows for improved fixation. Additionally, the Trigon Lapidus Wedge is the first implant to specifically reference lengthening in its FDA indication.
The Trigon Lapidus Wedge is a PEEK-OPTIMA HA Enhanced implant indicated for a first metatarsal-cuneiform lengthening arthrodesis. It is offered in three footprint sizes with various length restoring thicknesses, as well as variations in sagittal and transverse angle correction. Additionally, the system provides a jig that allows for frontal plane rotation, resulting in triplanar correction with the ability to restore or maintain length of the first metatarsal.
The Trigon Stand-Alone Subtalar Wedge is the first PEEK-OPTIMA HA Enhanced wedge for Subtalar Fusion in the market. The PEEK-OPTIMA HA Enhanced material, which has a modulus similar to bone, allows artifact-free imaging and provides an osteoconductive surface for bone ongrowth. With a 25mm diameter, the Subtalar Wedge offers correction heights ranging from 6mm to 16mm in parallel and angled options.
In the last four months, Nvision added four products with FDA clearance to its portfolio, with one currently in FDA review and two more scheduled for submission.
Source: Nvision Biomedical Technologies

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







