
 Copy to clipboard
Copy to clipboard 
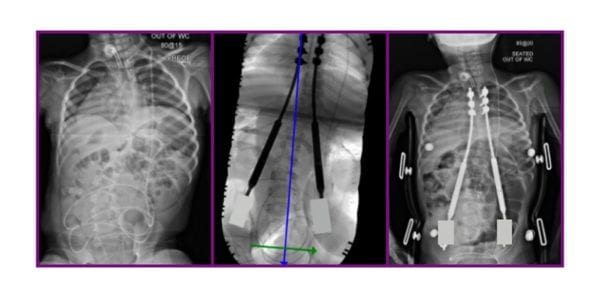
NuVasive voluntarily issued an Urgent Field Safety Notice to healthcare providers in the U.K. and Ireland to address concerns identified by The Medicines and Healthcare Products Regulatory Agency (MHRA) over continued use of the MAGEC device. No MAGEC System rods of any model number shall be implanted in the regions until further notice.
MAGEC, a metallic implant, is used to brace the spine during growth to minimize the progression of scoliosis. The Instructions for Use indicate that metallic implants can loosen, fracture, corrode, migrate or cause pain. Consistent with the IFU and prior communication, this can manifest in vivo as locking pin breakage, O-ring seal failure, metal wear debris and failure of the rod to distract. Localized tissue discoloration may result from use of the MAGEC rod. Further, earlier communications indicated that a 0.5% post-implantation separation of an actuator end cap component was observed in MAGEC System Model X rods.
While the system remains CE Marked, the MHRA and Health Products Regulatory Authority are undertaking a market surveillance review of MAGEC. NuVasive is working with the agencies throughout the process.
The system was first implanted in 2014 under Ellipse Technologies, which was acquired by NuVasive in early 2016. The company received FDA clearance for the system later that year. MAGEC X was launched in the U.K. in 2018.
NuVasive voluntarily issued an Urgent Field Safety Notice to healthcare providers in the U.K. and Ireland to address concerns identified by The Medicines and Healthcare Products Regulatory Agency (MHRA) over continued use of the MAGEC device. No MAGEC System rods of any model number shall be implanted in the regions until further notice.
...
NuVasive voluntarily issued an Urgent Field Safety Notice to healthcare providers in the U.K. and Ireland to address concerns identified by The Medicines and Healthcare Products Regulatory Agency (MHRA) over continued use of the MAGEC device. No MAGEC System rods of any model number shall be implanted in the regions until further notice.
MAGEC, a metallic implant, is used to brace the spine during growth to minimize the progression of scoliosis. The Instructions for Use indicate that metallic implants can loosen, fracture, corrode, migrate or cause pain. Consistent with the IFU and prior communication, this can manifest in vivo as locking pin breakage, O-ring seal failure, metal wear debris and failure of the rod to distract. Localized tissue discoloration may result from use of the MAGEC rod. Further, earlier communications indicated that a 0.5% post-implantation separation of an actuator end cap component was observed in MAGEC System Model X rods.
While the system remains CE Marked, the MHRA and Health Products Regulatory Authority are undertaking a market surveillance review of MAGEC. NuVasive is working with the agencies throughout the process.
The system was first implanted in 2014 under Ellipse Technologies, which was acquired by NuVasive in early 2016. The company received FDA clearance for the system later that year. MAGEC X was launched in the U.K. in 2018.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
ME
Mike Evers is a Senior Market Analyst and writer with over 15 years of experience in the medical industry, spanning cardiac rhythm management, ER coding and billing, and orthopedics. He joined ORTHOWORLD in 2018, where he provides market analysis and editorial coverage.






