
 Copy to clipboard
Copy to clipboard 
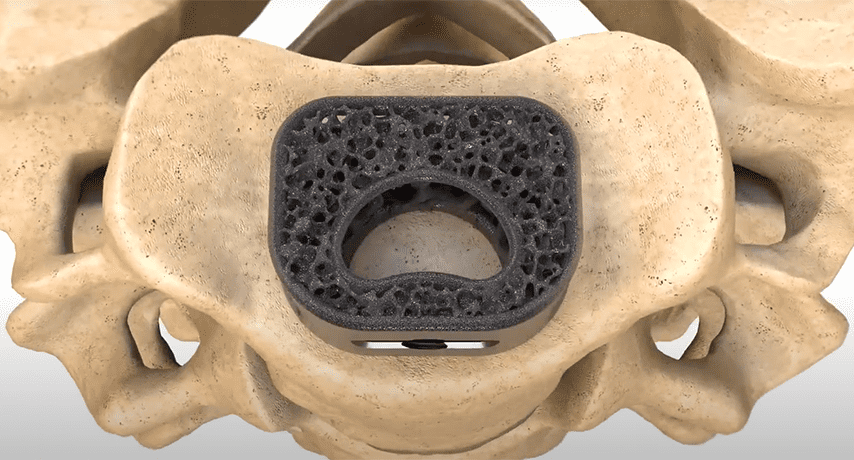
NuVasive received FDA 510(k) clearance for the use of its Modulus Cervical interbody implant with a bone void filler, thus enhancing the company’s C360 portfolio.
Attrax Putty is reportedly the first synthetic bone graft substitute to receive FDA-cleared Indications for Use in thoracolumbar interbody fusion spacers. Subsequently published evidence clinically validated the combination of NuVasive’s Advanced Materials Science solutions, Modulus XLIF and Attrax Putty, as having clinical and economic advantages over traditional, non-porous interbody implants and premium-priced biologics.
Source: NuVasive
NuVasive received FDA 510(k) clearance for the use of its Modulus Cervical interbody implant with a bone void filler, thus enhancing the company's C360 portfolio.
Attrax Putty is reportedly the first synthetic bone graft substitute to receive FDA-cleared Indications for Use in thoracolumbar interbody fusion spacers. Subsequently published...
NuVasive received FDA 510(k) clearance for the use of its Modulus Cervical interbody implant with a bone void filler, thus enhancing the company’s C360 portfolio.
Attrax Putty is reportedly the first synthetic bone graft substitute to receive FDA-cleared Indications for Use in thoracolumbar interbody fusion spacers. Subsequently published evidence clinically validated the combination of NuVasive’s Advanced Materials Science solutions, Modulus XLIF and Attrax Putty, as having clinical and economic advantages over traditional, non-porous interbody implants and premium-priced biologics.
Source: NuVasive

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







