
 Copy to clipboard
Copy to clipboard 
NuVasive (NUVA) grew its Advanced Materials Science™ (AMS) implant portfolio with commercial U.S. launch of the Modulus® XLIF® Dual Sided Plate, and FDA 510(k) clearances to market the Modulus ALIF and Cohere® TLIF-O devices.
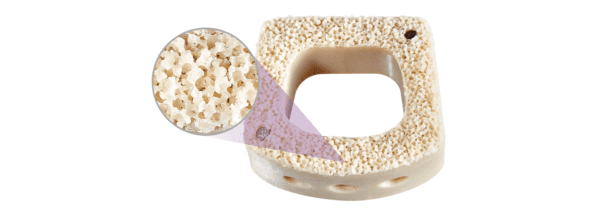
The AMS line includes proprietary surface and structural technologies designed to enhance the osseointegration and biomechanical properties of materials used in spine procedures. Modulus and Cohere implants are used in spinal fusion, combining porosity with the material properties of titanium and porous PEEK, respectively. The latter is depicted in the image of NUVA’s Cohere Cervical device, pictured above.
The Modulus XLIF Dual Sided Plate allows for two points of fixation in eXtreme Lateral Interbody Fusion. Modulus ALIF is a low-profile implant for use in both supine and lateral ALIF (XALIF), and is the company’s first AMS offering for the ALIF market. Cohere TLIF-O is a porous PEEK implant that includes NUVA’s single-step insert and rotate technique, as well as a lordotic design feature in the oblique plane, allowing surgeons to restore sagittal alignment of the spine while avoiding the introduction of an undesired coronal misalignment.
“With these new offerings, NuVasive strengthens its AMS portfolio to equip surgeons with best-in-class technology for a variety of thoracolumbar procedures,” said Matt Link, President of NuVasive. “NuVasive’s continued R&D initiatives underscore the company’s commitment to expand within key procedural spine segments and deliver differentiated technology to support better patient outcomes.”
NuVasive (NUVA) grew its Advanced Materials Science™ (AMS) implant portfolio with commercial U.S. launch of the Modulus® XLIF® Dual Sided Plate, and FDA 510(k) clearances to market the Modulus ALIF and Cohere® TLIF-O devices.
The AMS line includes proprietary surface and structural technologies designed to enhance the osseointegration and...
NuVasive (NUVA) grew its Advanced Materials Science™ (AMS) implant portfolio with commercial U.S. launch of the Modulus® XLIF® Dual Sided Plate, and FDA 510(k) clearances to market the Modulus ALIF and Cohere® TLIF-O devices.
The AMS line includes proprietary surface and structural technologies designed to enhance the osseointegration and biomechanical properties of materials used in spine procedures. Modulus and Cohere implants are used in spinal fusion, combining porosity with the material properties of titanium and porous PEEK, respectively. The latter is depicted in the image of NUVA’s Cohere Cervical device, pictured above.
The Modulus XLIF Dual Sided Plate allows for two points of fixation in eXtreme Lateral Interbody Fusion. Modulus ALIF is a low-profile implant for use in both supine and lateral ALIF (XALIF), and is the company’s first AMS offering for the ALIF market. Cohere TLIF-O is a porous PEEK implant that includes NUVA’s single-step insert and rotate technique, as well as a lordotic design feature in the oblique plane, allowing surgeons to restore sagittal alignment of the spine while avoiding the introduction of an undesired coronal misalignment.
“With these new offerings, NuVasive strengthens its AMS portfolio to equip surgeons with best-in-class technology for a variety of thoracolumbar procedures,” said Matt Link, President of NuVasive. “NuVasive’s continued R&D initiatives underscore the company’s commitment to expand within key procedural spine segments and deliver differentiated technology to support better patient outcomes.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







