
 Copy to clipboard
Copy to clipboard 
Diamond Orthopedic will supply faceted bone screws for the NuVasive Specialized Orthopedics (NSO) PRECICE® Plate for limb lengthening and reconstruction. PRECICE addresses pediatric applications or patients with small anatomy who cannot accommodate a lengthening device that is traditionally implanted within bone.
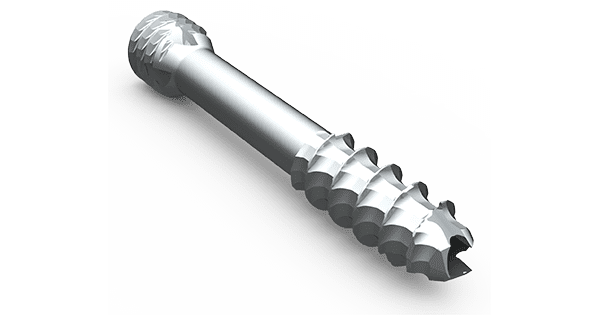
The agreement covers supply of locking and non-locking faceted threadform screws in a new system that is designed to affix to the outside of a patient’s bone to avoid potential damage to the growth plate. Diamond Orthopedic screws secure the plate during bone lengthening.
Diamond’s patented design allows surgeons to insert faceted screws into bone with reduced insertional torque and increased screw pullout. Studies have demonstrated an improved bone/screw interface versus traditional helical threadforms.
Diamond Orthopedic received FDA 510(k) clearance for the sterile faceted screw in 2018.
“This is a significant vote of confidence in the value proposition of faceted bone screws,” said Roy Bivens, CEO of Diamond Orthopedic. “Faceted bone screws offer unparalleled fixation, and we are especially proud to have the opportunity to bring this technology to pediatric cases as a part of NSO’s new PRECICE plating system that elevates the standard of care for pediatric patients with limb length discrepancy.”
Diamond Orthopedic will supply faceted bone screws for the NuVasive Specialized Orthopedics (NSO) PRECICE® Plate for limb lengthening and reconstruction. PRECICE addresses pediatric applications or patients with small anatomy who cannot accommodate a lengthening device that is traditionally implanted within bone.
The agreement covers...
Diamond Orthopedic will supply faceted bone screws for the NuVasive Specialized Orthopedics (NSO) PRECICE® Plate for limb lengthening and reconstruction. PRECICE addresses pediatric applications or patients with small anatomy who cannot accommodate a lengthening device that is traditionally implanted within bone.
The agreement covers supply of locking and non-locking faceted threadform screws in a new system that is designed to affix to the outside of a patient’s bone to avoid potential damage to the growth plate. Diamond Orthopedic screws secure the plate during bone lengthening.
Diamond’s patented design allows surgeons to insert faceted screws into bone with reduced insertional torque and increased screw pullout. Studies have demonstrated an improved bone/screw interface versus traditional helical threadforms.
Diamond Orthopedic received FDA 510(k) clearance for the sterile faceted screw in 2018.
“This is a significant vote of confidence in the value proposition of faceted bone screws,” said Roy Bivens, CEO of Diamond Orthopedic. “Faceted bone screws offer unparalleled fixation, and we are especially proud to have the opportunity to bring this technology to pediatric cases as a part of NSO’s new PRECICE plating system that elevates the standard of care for pediatric patients with limb length discrepancy.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.