
 Copy to clipboard
Copy to clipboard 
Results of the study, “Efficacy of a Standalone Microporous Ceramic vs. Autograft in Instrumented Posterolateral Spinal Fusion; a Multicenter, Randomized, Intra-patient Controlled, Non-inferiority Trial,” support the use of NuVasive’s (NUVA) Attrax® Putty as a standalone bone graft substitute for autograft in instrumented thoracolumbar posterolateral lumbar fusion (PLF). Aided by these results, NUVA received FDA 510(k) clearance for expanded use of Attrax Putty without autograft.
The trial enrolled 100 patients at four hospital sites. Following randomization, Attrax Putty was applied to one side of the spine and iliac crest autograft and local bone to the contralateral side, with each patient serving as their own control. Fusion was assessed at one year. CT scans showed fusion rates of 55% for the Attrax Putty side and 52% for the autograft side, with an overall fusion rate of 71% of levels. Attrax Putty alone successfully demonstrated non-inferior fusion performance compared to autograft in instrumented PLF.
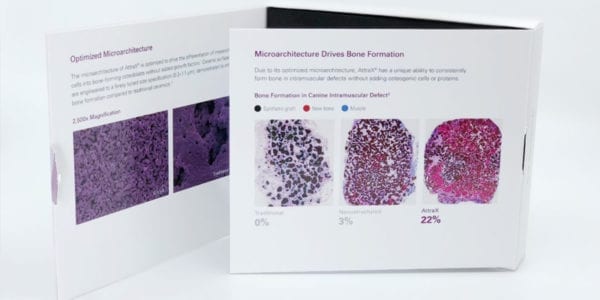
Attrax Putty is a synthetic, bioactive and osteoconductive bone void filler for the repair of bone defects. Its surface microarchitecture provides an environment for bone formation without added cells or growth factors. The moldable material is resorbed and replaced by bone during healing.
Results of the study, "Efficacy of a Standalone Microporous Ceramic vs. Autograft in Instrumented Posterolateral Spinal Fusion; a Multicenter, Randomized, Intra-patient Controlled, Non-inferiority Trial," support the use of NuVasive's (NUVA) Attrax® Putty as a standalone bone graft substitute for autograft in instrumented thoracolumbar...
Results of the study, “Efficacy of a Standalone Microporous Ceramic vs. Autograft in Instrumented Posterolateral Spinal Fusion; a Multicenter, Randomized, Intra-patient Controlled, Non-inferiority Trial,” support the use of NuVasive’s (NUVA) Attrax® Putty as a standalone bone graft substitute for autograft in instrumented thoracolumbar posterolateral lumbar fusion (PLF). Aided by these results, NUVA received FDA 510(k) clearance for expanded use of Attrax Putty without autograft.
The trial enrolled 100 patients at four hospital sites. Following randomization, Attrax Putty was applied to one side of the spine and iliac crest autograft and local bone to the contralateral side, with each patient serving as their own control. Fusion was assessed at one year. CT scans showed fusion rates of 55% for the Attrax Putty side and 52% for the autograft side, with an overall fusion rate of 71% of levels. Attrax Putty alone successfully demonstrated non-inferior fusion performance compared to autograft in instrumented PLF.
Attrax Putty is a synthetic, bioactive and osteoconductive bone void filler for the repair of bone defects. Its surface microarchitecture provides an environment for bone formation without added cells or growth factors. The moldable material is resorbed and replaced by bone during healing.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







