
 Copy to clipboard
Copy to clipboard 
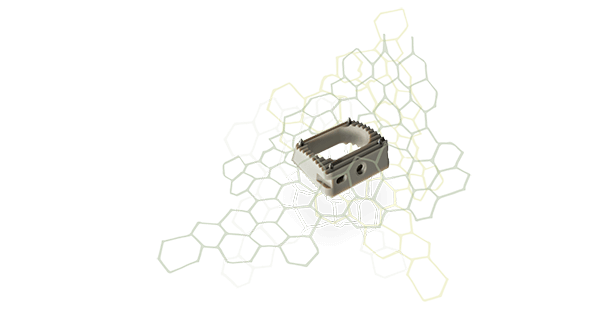
NGMedical introduced the BEE® HA cage within CE Mark-approved regions. BEE HA is an anatomically shaped cervical interbody made of PEEK-OPTIMA™ HA Enhanced developed by Invibio Biomaterial Solutions.
BEE HA was developed to maintain elasticity that is similar to bone and radiolucency of the cage body. Hydroxyapatite is fully integrated into, as opposed to coated on, the PEEK-OPTIMA matrix.
NGMedical has submitted the device for FDA 510(k) clearance.
NGMedical’s other launched products include the MOVE-C® cervical disc and the BEE 3D Ti Cervical Interbody Cage.
NGMedical introduced the BEE® HA cage within CE Mark-approved regions. BEE HA is an anatomically shaped cervical interbody made of PEEK-OPTIMA™ HA Enhanced developed by Invibio Biomaterial Solutions.
BEE HA was developed to maintain elasticity that is similar to bone and radiolucency of the cage body. Hydroxyapatite is fully integrated...
NGMedical introduced the BEE® HA cage within CE Mark-approved regions. BEE HA is an anatomically shaped cervical interbody made of PEEK-OPTIMA™ HA Enhanced developed by Invibio Biomaterial Solutions.
BEE HA was developed to maintain elasticity that is similar to bone and radiolucency of the cage body. Hydroxyapatite is fully integrated into, as opposed to coated on, the PEEK-OPTIMA matrix.
NGMedical has submitted the device for FDA 510(k) clearance.
NGMedical’s other launched products include the MOVE-C® cervical disc and the BEE 3D Ti Cervical Interbody Cage.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







