
 Copy to clipboard
Copy to clipboard 
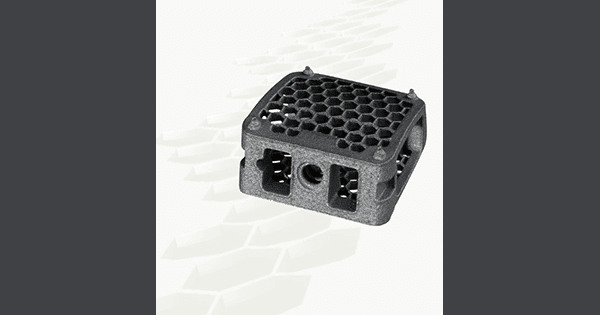
NGMedical received FDA 510(k) clearance to market its additively manufactured titanium cervical BEE® cage.
The honeycomb structure of BEE supports bony ingrowth and demonstrates the reduced use of titanium minimizing risks of x-ray artefacts, while offering a very large graft space.
“This is an important step for NGMedical and allows us to start our active market presence in the USA. We are proud having received the FDA clearance based on the innovative design introduced to the OUS market in 2020. Our team did a great job in cooperation with MRC Global,” said Nino Weiland, Operations Manager of NGMedical.
NGMedical received FDA 510(k) clearance to market its additively manufactured titanium cervical BEE® cage.
The honeycomb structure of BEE supports bony ingrowth and demonstrates the reduced use of titanium minimizing risks of x-ray artefacts, while offering a very large graft space.
"This is an important step for NGMedical and allows us...
NGMedical received FDA 510(k) clearance to market its additively manufactured titanium cervical BEE® cage.
The honeycomb structure of BEE supports bony ingrowth and demonstrates the reduced use of titanium minimizing risks of x-ray artefacts, while offering a very large graft space.
“This is an important step for NGMedical and allows us to start our active market presence in the USA. We are proud having received the FDA clearance based on the innovative design introduced to the OUS market in 2020. Our team did a great job in cooperation with MRC Global,” said Nino Weiland, Operations Manager of NGMedical.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







