
 Copy to clipboard
Copy to clipboard 
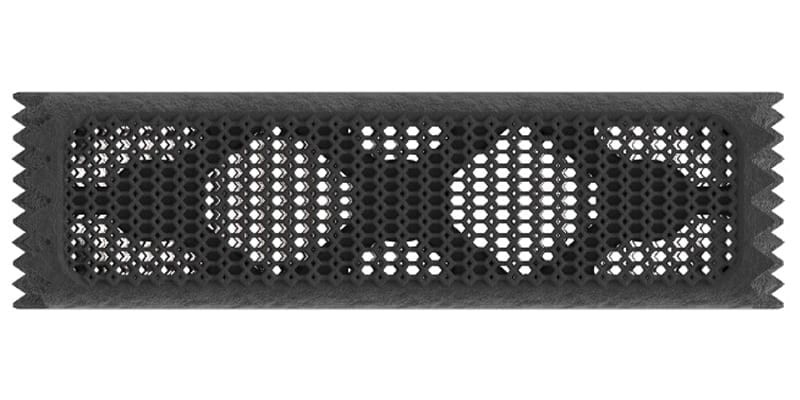
Nexxt Spine received FDA 510(k) clearance of the 3D-printed Nexxt Matrixx® Corpectomy System, and commenced product launch.
Corpectomy features placement of varying pore sizes (300μ-700μ) along with macro/microsurfacing and bone biology relevance. A continuous lumen allows the cage to be packed with the surgeon’s chosen biologic from endplate to endplate.
Nexxt Matrixx Corpectomy System is cleared for use in the cervical and thoracolumbar spine to replace a diseased, collapsed, damaged or unstable vertebral body due to tumor, osteomyelitis, and trauma, or for reconstruction following corpectomy. A roughened surface and inferior/superior teeth provide initial stabilization.
“Here at Nexxt Spine we are committed to bringing exceptionally innovative products to the market,” says President, Andy Elsbury. “The extension of our Nexxt Matrixx® line with the Corpectomy System has been highly anticipated by our surgeon network, who consistently experience superior clinical outcomes. We are excited to expand this network and positively enhance the lives of more patients.”
Nexxt Spine received FDA 510(k) clearance of the 3D-printed Nexxt Matrixx® Corpectomy System, and commenced product launch.
Corpectomy features placement of varying pore sizes (300μ-700μ) along with macro/microsurfacing and bone biology relevance. A continuous lumen allows the cage to be packed with the surgeon's chosen biologic from endplate...
Nexxt Spine received FDA 510(k) clearance of the 3D-printed Nexxt Matrixx® Corpectomy System, and commenced product launch.
Corpectomy features placement of varying pore sizes (300μ-700μ) along with macro/microsurfacing and bone biology relevance. A continuous lumen allows the cage to be packed with the surgeon’s chosen biologic from endplate to endplate.
Nexxt Matrixx Corpectomy System is cleared for use in the cervical and thoracolumbar spine to replace a diseased, collapsed, damaged or unstable vertebral body due to tumor, osteomyelitis, and trauma, or for reconstruction following corpectomy. A roughened surface and inferior/superior teeth provide initial stabilization.
“Here at Nexxt Spine we are committed to bringing exceptionally innovative products to the market,” says President, Andy Elsbury. “The extension of our Nexxt Matrixx® line with the Corpectomy System has been highly anticipated by our surgeon network, who consistently experience superior clinical outcomes. We are excited to expand this network and positively enhance the lives of more patients.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







