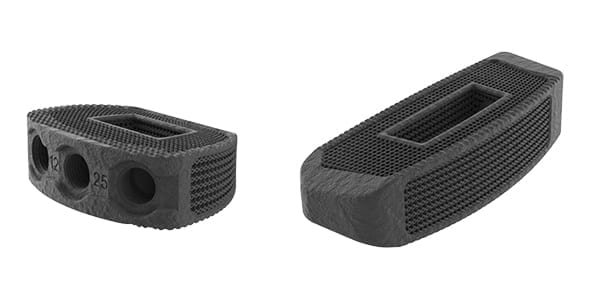






Nexxt Spine was granted FDA 510(k) clearance to market ALIF and Lateral lumbar systems. Alpha launch is slated for 3Q20, with integrated plate systems coming in 4Q20.
Both systems feature Nexxt Matrixx® 3D-printed titanium technology to create the desired pore size and geometry, and have a modulus of elasticity lower than PEEK, a microroughened surface and a 75% porous lattice.
“We are thrilled to be releasing these two powerhouses this year,” said President Andy Elsbury. “Our engineering and 3D printing manufacturing teams has been working diligently to simultaneously develop and clear the two products after considerable surgeon demand for Nexxt Spine quality clinical outcomes for ALIF and Lateral approaches.”
Nexxt Spine was granted FDA 510(k) clearance to market ALIF and Lateral lumbar systems. Alpha launch is slated for 3Q20, with integrated plate systems coming in 4Q20.
Both systems feature Nexxt Matrixx® 3D-printed titanium technology to create the desired pore size and geometry, and have a modulus of elasticity lower than PEEK, a...
Nexxt Spine was granted FDA 510(k) clearance to market ALIF and Lateral lumbar systems. Alpha launch is slated for 3Q20, with integrated plate systems coming in 4Q20.
Both systems feature Nexxt Matrixx® 3D-printed titanium technology to create the desired pore size and geometry, and have a modulus of elasticity lower than PEEK, a microroughened surface and a 75% porous lattice.
“We are thrilled to be releasing these two powerhouses this year,” said President Andy Elsbury. “Our engineering and 3D printing manufacturing teams has been working diligently to simultaneously develop and clear the two products after considerable surgeon demand for Nexxt Spine quality clinical outcomes for ALIF and Lateral approaches.”


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







