
 Copy to clipboard
Copy to clipboard 
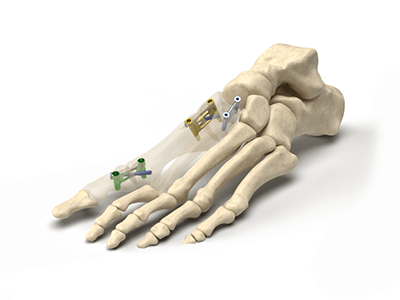
Nextremity Solutions will begin commercialization of its StealthFix™ intraosseous fixation platform for use in upper and lower extremities.
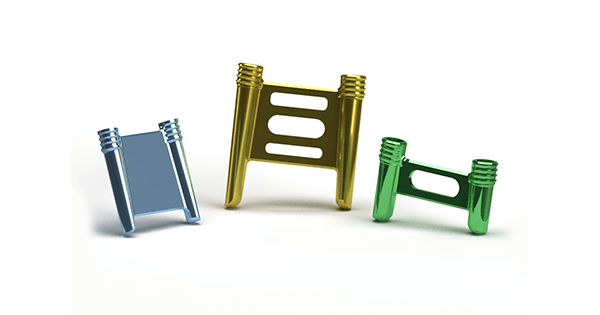
StealthFix is offered for the treatment of arthrodesis, osteotomies and bone fractures, and presents an alternative to bone plates, staples and screws, which are placed on bone. StealthFix is completely implanted within the bone, minimizing complications due to hardware prominence.
The StealthFix platform features cross-joint construction of the implant. This allows for evenly distributed compression across the fracture or osteotomy site. In finite element analysis, as well as biomechanical testing, StealthFix implants displayed more strength, stronger bone-to-bone apposition and reduced gap formation compared to screw and bone plate systems.
Ryan Schlotterback, Chief Technology Officer for Nextremity Solutions, said, “StealthFix has achieved 510(k) clearance for use in the fixation of fractures, fusions, and reconstructions in the lower and upper extremity. …The system’s simplicity of insertion, the intraosseous, zero-profile nature of the implant, and the stability and fixation it provides will be a great addition to a partner’s portfolio.”
Nextremity Solutions will begin commercialization of its StealthFix™ intraosseous fixation platform for use in upper and lower extremities.
StealthFix is offered for the treatment of arthrodesis, osteotomies and bone fractures, and presents an alternative to bone plates, staples and screws, which are placed on bone. StealthFix is...
Nextremity Solutions will begin commercialization of its StealthFix™ intraosseous fixation platform for use in upper and lower extremities.
StealthFix is offered for the treatment of arthrodesis, osteotomies and bone fractures, and presents an alternative to bone plates, staples and screws, which are placed on bone. StealthFix is completely implanted within the bone, minimizing complications due to hardware prominence.
The StealthFix platform features cross-joint construction of the implant. This allows for evenly distributed compression across the fracture or osteotomy site. In finite element analysis, as well as biomechanical testing, StealthFix implants displayed more strength, stronger bone-to-bone apposition and reduced gap formation compared to screw and bone plate systems.
Ryan Schlotterback, Chief Technology Officer for Nextremity Solutions, said, “StealthFix has achieved 510(k) clearance for use in the fixation of fractures, fusions, and reconstructions in the lower and upper extremity. …The system’s simplicity of insertion, the intraosseous, zero-profile nature of the implant, and the stability and fixation it provides will be a great addition to a partner’s portfolio.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.