
 Copy to clipboard
Copy to clipboard 
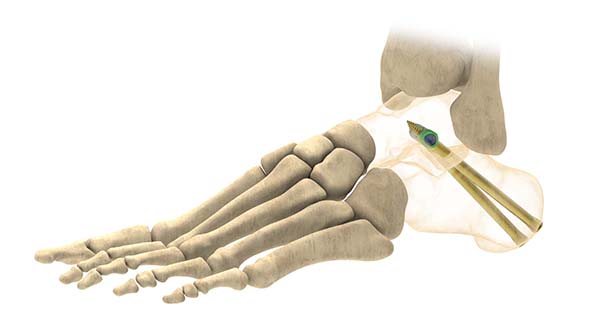
Nextremity Solutions received FDA 510(k) clearance to market its upcoming InCore® Subtalar System.
Expanding on the InCore brand, preceded by the InCore Lapidus System and InCore TMT System, InCore Subtalar provides surgeons with a three-part intraosseous fixation option for subtalar joint arthrodesis.
“This is the fourth 510(k) clearance that was achieved in 2021 by Nextremity Solutions and is evidence of our culture of innovation and teamwork. As these technologies enter the marketplace in 2022, it once again proves out our i3 Strategic Solutions product development and commercialization strategy. I couldn’t be more proud of this team of talented and driven individuals,” added President & CEO, Rod K. Mayer.
The Nextremity Solutions InCore Subtalar System is indicated for reduction and internal fixation of arthrodeses, osteotomies, and nonunions of the bones and joints of the foot. The three-part construct is specifically intended for internal fixation for Subtalar Joint Arthodesis (also known as Subtalar Joint Fusion).
Source: Nextremity Solutions, Inc.
Nextremity Solutions received FDA 510(k) clearance to market its upcoming InCore® Subtalar System.
Expanding on the InCore brand, preceded by the InCore Lapidus System and InCore TMT System, InCore Subtalar provides surgeons with a three-part intraosseous fixation option for subtalar joint arthrodesis.
“This is the fourth 510(k) clearance that...
Nextremity Solutions received FDA 510(k) clearance to market its upcoming InCore® Subtalar System.
Expanding on the InCore brand, preceded by the InCore Lapidus System and InCore TMT System, InCore Subtalar provides surgeons with a three-part intraosseous fixation option for subtalar joint arthrodesis.
“This is the fourth 510(k) clearance that was achieved in 2021 by Nextremity Solutions and is evidence of our culture of innovation and teamwork. As these technologies enter the marketplace in 2022, it once again proves out our i3 Strategic Solutions product development and commercialization strategy. I couldn’t be more proud of this team of talented and driven individuals,” added President & CEO, Rod K. Mayer.
The Nextremity Solutions InCore Subtalar System is indicated for reduction and internal fixation of arthrodeses, osteotomies, and nonunions of the bones and joints of the foot. The three-part construct is specifically intended for internal fixation for Subtalar Joint Arthodesis (also known as Subtalar Joint Fusion).
Source: Nextremity Solutions, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







